Global Stem Cells Group Names Enrique Testart, M.D. Chief Medical Officer
Tuesday, January 5, 2016
Global Stem Cells Group has named Enrique Testart, M.D., to the position of Chief Medical Officer for the Miami-headquartered biotech company.
MIAMI, Dec. 22, 2015—Global Stem Cells Group CEO Benito Novas announced the appointment of Enrique Testart, M.D. as Chief Medical Officer (CMO) of the international biotech company. Testart is a surgeon specializing in child trauma microsurgery. Testart is also a medical entrepreneur and founder of Consortia Innovas, SA in Santiago, Chile, a company dedicated to consulting and clinical health management for clinical management firms and research and development-oriented planners in the latest treatments in regenerative medicine as they become available.
Testart is the latest addition to GSCG’s recent string of high profile appointments including Novas as company CEO, Kipp Van Camp, D.O. as Chief Operating Officer, and Alfredo Hoyos, M.D. as head of the GSCG Advisory Board. According to Novas, the appointments mark Global Stem Cell Group’s exponential growth in Latin American and worldwide.
Testart, a native of Santiago, earned a medical degree from the University of Chile in Santiago, and began practicing medicine in a rural Chile hospital. He returned to Santiago to accept a fellowship at the Dr. Exequiel González Cortéz Hospital, part of Chile’s public health network.
He traveled to northeastern France in the 1980s to study orthopedics under the direction of Prof. Dr. Jean Paul Metaizeau. Testart remained in the region to specialize in microsurgery at Belle Isle Mertz Hospital, and while in France he began treating children with congenital musculoskeletal diseases such as osteogenesis imperfecta (brittle bone disease). Upon returning to Chile, Testart resumed his education to further study the embrochage centro médullaire élastique stable (E.C.M.E.S.) techniques he had studied under Metaizeau, which have become a standard worldwide for treating children with normal fractures, as well as those suffering from the spontaneous fractures caused by brittle bone disease.
Testart established a private medical practice in Santiago, founded the Association of Relatives of Patients with Osteogenesis Imperfecta, and began studying cell therapy research underway in Scandinavia. In 2000, Testart was tracking treatments with the latest technology in regenerative therapy, cell regeneration and isolation of mesenchymal stem cells from bone marrow while developing techniques and protocols to manage musculoskeletal disorders.
He trained further at the Domenikus Krankennouse Clinic in Germany and XCell Centers in London, Netherlands and Germany. He expanded the institution’s practice to Chile, acting as a consultant to various health organizations and doctors in a joint search for innovative medical solutions for orthopedic diseases and disorders. Gradually, he incorporated elements of regenerative medicine therapies into his own protocols, and went on to become the founder of the Institute of Advanced Cell Medicine and Bioengineering SAP, dedicated to the training, implementation and application of therapy tissue regeneration.
Testart is a consultant for the German Clinic of Santiago, San Cristobal Clinic Foundation, Stem Cells and Stem Cells Center at the Providence Marriott Center in Santiago, and is responsible for compiling reports and presenting them in Chile and Latin American countries.
After completing internships in the US and Mexico, Testart established The Institute, a group of organizations designed to meet the different needs of a new generation of medical consumers. With more than 20 years in the public and private sectors, the group of companies specializes in designing custom B2B and B2C programs for each central or regional organization model, providing innovative services and diagnostic accuracy to communities throughout Chile according to local needs.
Testart represents all Global Stem Cells Group divisions and programs in Chile, including patient recruitment through Cellgenic, and all responsibilities related to the sale of equipment disposable through Adimarket. He also oversees medical training and certification through Stem Cell Training Inc., with centers in three regions of Chile and plans to double that number in 2016.
Cellgenic, Stem Cell Training, Inc. and Adimarket are all subsidiaries of Global Stem Cells Group.
For more information, visit the Global Stem Cells Group website, email bnovas@stemcellsgroup.com, or call ![]() 305-560-5337.
305-560-5337.
About the Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies. each dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions.
With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Groups corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCGs six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
###
- Published in Press Releases
No Comments
Global Stem Cells Group Appoints Daniça Goleš Chief International Marketing Officer
Tuesday, January 5, 2016
MIAMI, Dec. 22, 2015—Global Stem Cells Group announced the appointment of Daniça Goleš to the position of chief international marketing officer for the biotech company. Goleš, who has served in the same capacity for the GSCG headquarters in Chile since 2014, is a specialist in sociology and coaching with a concentration in medicine and surgery, and has 20 years’ experience in medical marketing, management and strategic communications.
Prior to joining Global Stem Cells Group’s team in Chile, Goleš was the director of strategic communication management in the two largest university hospitals in Chile, leading the department for seven years at the University of Chile and another seven years at the Pontifical Catholic University of Chile.
Goleš developed two private strategic consulting practices for the medical and surgical areas of organ transplant teams, digestive surgery, bariatric, rare diseases, orthopedics and plastic surgery for Chile’s most important advocates.
She was a founding partner of Healthvision Ltd., Coach Pacients SA, and Communication and Medicine Ltd. Both companies engaged in the management of medical and health content and validating reliable sources for the media, development and production of television programs and developing business models for clinical management. Goleš establshed policy for improved patient information and improved relationships between the organization’s professionals and end users.

Since beginning her training in 2000 on Chile’s first steps in cell therapy, Goleš developed management models for regenerative medicine patients. Next, she completed internships in Germany and Chicago with stem cell development groups. She is a member of the International Society of Cell Therapy and the International Society for Stem Cell Research (ISSR).
In 2012, she received the Robert Wood Johnson Foundation (Johnson & Johnson) Community Health Leaders award, which honors advocates in the health care industry who have made a difference in improving the health and access to care for patients in underserved populations.
Goleš graduated from the Professional Institute of Santiago and completed her post graduate studies at the Universidad Alberto Hurtado in Santiago, Chile in 2002 with a diploma in Communications and New Technologies. She subsequently completed her masters degree in Psychology and Coaching at the University of Chile Institute of Communications with concentrations in Clinical Units Top Management, strategic planning at Les Halles, Paris and brand management and Internet Domains at the University of Chile.
“We are very happy that Daniça is bringing her skills to our international efforts at a time when the company is growing exponentially worldwide,” says Global Stem Cells Group CEO Benito Novas. “Danica did a remarkable job for us in Chile, and now we can benefit from her talents at the international level.”
For more information, visit the Global Stem Cells Group website, Email bnovas@stemcellsgroup.com, or call ![]() 305-560-5337.
305-560-5337.
About the Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions.
With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Groups corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCGs six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.

- Published in Press Releases
Essential Steps for Regenerative Medicine Practitioners
Monday, January 4, 2016
Comprehensive Stem Cell Training
Global Stem Cells Group (GSCG) offers an intensive two-day stem cell training course designed to equip practitioners with the skills to implement cutting-edge regenerative therapies in their clinics. The training includes hands-on experience in techniques such as precision mini lipo-aspiration for fat tissue collection and bone marrow harvesting via the iliac crest.
Didactic Lectures and Practical Application
Participants receive didactic lectures covering stem cell structure, function, and treatment protocols. Practical sessions involve the isolation and harvesting of stem cells from both fat tissue and bone marrow, as well as the extraction of platelet-rich plasma from peripheral blood. Live demonstrations on actual patients ensure thorough understanding and proficiency in performing these procedures.
Certification and Support
Upon completion, GSCG provides written protocols, consent forms, and necessary documentation for seamless integration of stem cell therapies into clinical practice. The course is fully accredited, offering 16 continuing education credits across multiple categories from three universities. Participants also have the opportunity to engage in Institutional Review Board (IRB) clinical studies to further their expertise.
FDA-Approved Products and Partnerships
GSCG collaborates with FDA 510K-approved partners like Emcyte to provide reliable bone marrow and platelet-rich plasma kits. These systems ensure precise processing of bone marrow aspirates, yielding concentrated growth factors and platelets suitable for therapeutic use.
Adipose-Derived Stem Cell Kits
Fat tissue serves as a rich source of mesenchymal stem cells, offering significantly higher yields compared to bone marrow. GSCG’s adipose-derived stem cell kits support the treatment of degenerative diseases and tissue replenishment post-injury. These multipotential progenitor cells are angiogenic and vasculogenic, making them effective in conditions involving ischemia and vascular regeneration.
Laboratory and Equipment Support
GSCG operates a state-of-the-art laboratory in Santiago, Chile, producing high-quality reagents and isolation kits under stringent good manufacturing practices (GMP). Adimarket.net offers a range of products including centrifuges and medical devices essential for implementing stem cell therapies in clinical settings. Training covers the selection and use of equipment required for various therapeutic techniques.
- Published in Corporate News / Blog
Global Stem Cells Group and Adimarket Announces Agreement with MyStem to Distribute Stem Cell Technology Products in Latin America
Tuesday, December 22, 2015
Adimarket , a division of Global Stem Cells Group, has signed an agreement to distribute Mystem stem cell technology in Latin America
MIAMI (PRWEB) December 06, 2015
Adimarket, Inc., a leading provider of in-office regenerative medicine solutions, has reached an agreement with MyStem to promote and distribute their stem cell technology products throughout Latin America.
MyStem develops and manufactures regenerative products for use in orthobiologics (ortho, trauma, spine), sports medicine, plastic surgery, aesthetics and dermatology treatments.
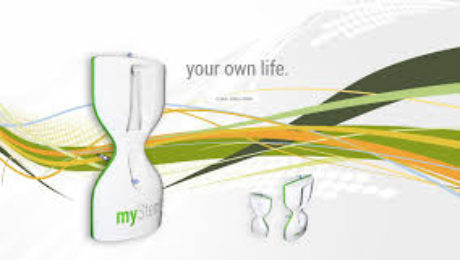
MyStem products include the innovative adipose-derived regenerative fraction device MyStem X2, a disposable collection and separation system for autologous adipose-derived mesenchymal stem cells (AdMSC). The MyStem device collects regenerative fraction without any extensive tissue manipulation, according to requirements established under international laws, in a completely safe closed sterile system. The regenerative fraction separation process is based on cells intrinsic parameters in a liquid fraction according to fluidic laws.
After the separation process, a concentrated regenerative cells fraction suspension is ready for use in a one-step procedure. MyStem offers an enhanced method for isolating and quickly expanding a robust population of mesenchymal stem cells (MSCs) derived from lipoaspirate in less than 30 minutes. This isolation process yields a generous population of ASCs (~100,000 cells per 100 ml of blood/saline collected from sonicated lipoaspirate) with differentiation potential, characteristic cell surface markers, and proliferative lifespan indistinguishable from MSCs extracted from bone marrow (BMSCs) or conventionally processed ASCs.(1,2 ).
The MyStem process is simple, with no centrifuge needed. Quality sampling is not operator dependent, and collects up to 1,000 times more than the bone marrow MSC + growth factors.
MyStem Managing Director Pier Ivona calls the new alliance with Adimarket a natural fit, since both companies share a deep commitment to getting critical regenerative medicine tools to the clinicians in Latin America who need them.
“Global Stem Cells Group and MyStem are both pioneers in stem cell research and innovation, and we share the same goal of making stem cell therapies available globally,” Ivona says. “We believe that this is the perfect time to team with Global Stem Cells Group and its Adimarket division to distribute MyStem technology throughout Latin America’s fast-growing medical community.”
The collaboration between Global Stem Cells Group and MyStem reflects one more step toward GSCG’s commitment to expanding its presence in communities that need and deserve access to cutting-edge regenerative medicine, not only in Latin America but also worldwide.
For more information, visit the Adimarket website, email info(at)adimarket(dot)net, or call ![]() +1 305 560 5337.
+1 305 560 5337.
About Adimarket:
Adimarket, Inc., a division of the Global Stem Cells Group, is a cost-competitive online marketplace for quality regenerative medicine equipment and supplies for physicians and health care professionals.
Adimarket was founded to provide physicians and other health care professionals the tools they need to practice regenerative medicine in a medical office setting. Motivated by a firm belief in the impact the practice of stem cell medicine can have when dispensed in a doctor’s office, Adimarket provides physicians with the tools they need to provide patients with cutting edge treatments.
 Adimarket’s experienced customer service representatives provide valuable guidance and advice regarding products relevant to individual practices.
Adimarket’s experienced customer service representatives provide valuable guidance and advice regarding products relevant to individual practices.
About the Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About MyStem:
Wilmington, Delaware-based MyStem is dedicated to contributing to human welfare through the design, manufacture, and sale of regenerative medicine instruments or appliances designed to alleviate pain and restore health. MyStem specializes in regenerative medicine treatments for: spine, ortho, sports medicine, burn and wound healing, CMF (cranio maxillo facial), chronic wounds and diabetic foot wounds.
###
- Published in Press Releases
Stemlab.com Founder and Prominent Plastic Surgeon Alfredo Hoyos to Head Global Stem Cells Group faculty
Tuesday, December 22, 2015
MIAMI, Oct. 5, 2015— Global Stem Cells Group, Inc. has named Stemlab.com founder and prominent plastic surgeon Alfredo Hoyos, M.D. as permanent head of the GSCG Faculty. Hoyos has been an active member of the GSCG team since signing on as an exclusive representative for the stem cell leader’s Colombian territory in September, 2014.
The Global Stem Cells Group / Stem Cell Training Faculty consists of a distinguished board of physicians, all experts in stem cell and regenerative medicine. The faculty reviews, updates and makes decision pertaining to the training programs offered through the Stem Cell Training division of Global Stem Cells Group. Faculty members also share the latest hand-on techniques and technologies with physicians, nurses, and other health care professionals through SCT’s instructional stem cell training courses.
 Hoyos divides his time between practices in Miami and Bogota, Colombia.
Hoyos divides his time between practices in Miami and Bogota, Colombia.
Hoyos earned his medical degree at the University of Rosario and completed his residency in plastic surgery at Rosario – Saint Joseph Hospital in Bogotá, Colombia. He later founded Stemlab to further the development of aesthetic techniques using stem cell (stromal) derived from adipose tissue.
Stemlab is currently conducting extensive research in regenerative medicine in an effort to establish stem cell treatments that can repair damaged tissue in living organisms, using natural biological mechanisms that are proving useful in auto repair processes on damaged tissue.
Hoyos is the inventor of the High Definition Liposculpture (HDL) and Dynamic Definition at Lipoplasty (4D) techniques as well as other advanced techniques that focus on body contouring.
Author of several scientific articles that discuss innovations and new technologies that work on body contour, Hoyos is also the inventor of proprietary tools and applications of plastic surgery devices.
To learn more, visit the Global Stem Cells Group website, email bnovas@steemcellsgroup.com, or call ![]() +1 305 560 5337.
+1 305 560 5337.
About the Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
- Published in Press Releases
Global Stem Cells Group, Inc. Announces New Regenerative Medicine Software App StemData to launch in 2016
Tuesday, December 22, 2015
MIAMI, Sept. 21, 2015—Global Stem Cells Group, in collaboration with medical software company Datamed SA Chile, has announced the plans to launch StemData, the first cloud-based electronic medical record system specifically designed for regenerative medicine practitioners.
The new StemData system will come programmed with the latest point of care regenerative medicine protocols and follow-up procedures to make it easy for stem cell practitioners to document patient progress and gather important data to establish the safety and efficacy of stem cell treatments in an doctor’s office environment. The system app will be marketed exclusively through Global Stem Cells Group and will be provided free of charge to affiliate physicians in the new Stem Cell Center network.

The cloud-based, server-less StemData solution will be accessible via web, iPad, iPhone, Google Glass and Apple Watch. Global Stem Cells Group and Datamed have utilized the development of the latest electronic medical record (EMR) systems to bring intelligent EMR technology to regenerative medicine specialists.
The StemData app will help regenerative medicine physicians increase efficiencies in their medical practices while improving both treatment and business outcomes. The app will intuitively adapt to each individual practitioner’s unique practice preferences.
To learn more, visit the Global Stem Cells Group website, email bnovas(at)regenestem(dot)com, or call ![]() 305-224-1858.
305-224-1858.
About the Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
- Published in Press Releases
Global Stem Cells Group, Inc. Announces Strategic Alliance with Cellus
Tuesday, December 22, 2015
Global Stem Cells Group has announce a new alliance with Santiago, Chile-based Cellus, a stem cell company dedicated to research, development and commercialization of therapeutic services and custom biotech products in regenerative medicine
MIAMI, FLA. (PRWEB) SEPTEMBER 28, 2015
Global Stem Cells Group has announced a new strategic alliance with Cellus to promote clinical research, physician training and patient treatment in Chile. Based in Santiago, Chile, Cellus is a stem cell company and full GMP lab dedicated to research, development and commercialization of therapeutic services and custom biotech products in the field of regenerative medicine.

Cellus’s state of the art medical facility, located at the Santiago Marriot, includes conference rooms with views to its cell culture lab and a full, cutting edge operating room for performing stem cell procedures. Cellus is managed by a multi-disciplinary team with extensive clinical experience and biopharmaceutical production, operating under the highest standards and international requirements for quality and safety. Cellus is continuously working to develop effective and innovative regenerative medicine solutions using autologous cellular therapies and biological products obtained from and administered to each individual patient.
“We are very pleased about our new alliance with Global Stem Cells Group,” says Cellus Commercial Manager Carlos Girardi. “We plan to leverage this alliance to further our clinical research, and to boost physician training here in Chile.
“This is a great opportunity to prove how valuable a collaboration between two international stem cell forces like Cellus and GSCG can be.”
Global Stem Cells Group, Chile CEO Enrique Testart agrees, adding that the new collaboration between the two companies will help GSCG establish itself as the leader in regenerative medicine therapies in Chile.
“Both Cellus and Global Stem Cells Group bring extraordinary insight, knowledge and experience in stem cell medicine to the table, and together we can accomplish great things,” Testart says. “I’m very excited to work with the excellent team at Cellus.”
The collaboration is part of Global Stem Cells Group’s push to highlight Chile’s appeal as a destination for patients seeking stem cell therapies for a variety of conditions, and as part of its ongoing commitment to expand the reach of stem cell treatments throughout Latin America.
To learn more, visit the Global Stem Cells Group website, email bnovas(at)regenestem(dot)com, or call ![]() 305-224-1858.
305-224-1858.
About the Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV. https://finance.yahoo.com/quote/mssv/
About Cellus:
Cellus is dedicated to research, development and commercialization of therapeutic services and custom biotech products in the field of regenerative medicine. Their multidisciplinary team of stem cell experts focus on the prevention and reversal of impairment in the skin of patients with traumatic injuries, skin diseases or symptoms of aging.
Their state-of-the-art facility in Santiago features a full laboratory and showcases the company’s extensive clinical expertise and biopharmaceutical production capabilities.
###
- Published in Press Releases
Global Stem Cells Group, Inc. Announces Collaboration with VidaCel Laboratories and Stem Cell Bank, Chile
Tuesday, December 22, 2015
Global Stem Cells Group announces a new alliance with Santiago, Chile-based VidaCel Laboratories, Latin America’s oldest and largest cryopreserved umbilical cord blood bank.
MIAMI (PRWEB) SEPTEMBER 25, 2015
Global Stem Cells Group has announced a new collaboration with Santiago, Chile-based VidaCell Laboratories, Latin America’s oldest and largest cryopreserved umbilical cord cell bank. The alliance is focused on creating new protocols for cryopreservation of cord blood, cord tissue, adipose tissue and dental pulp stem cells, and the culture and expansion of cell colonies.
The GSCG / VidaCel alliance will provide both companies broader access to the newest stem cell technologies for application in new territories. With Global Stem Cells Group’s growing presence in Latin America, the collaboration with VidaCel will eliminate the potential for significant delays in collecting and processing stem cells, which can result in a loss of cell viability.
According to Global Stem Cells Group CEO Benito Novas, the rules of the National Program for Bone Marrow Donation in the U.S. provide the guidelines used by VidaCel for processing cord blood cells within 24 hours.
“VidaCel’s location in Chile guarantees immediate availability of cells,” Novas says.
“Their process of collecting, processing and preserving stem cells is performed under the strictest standards of biosecurity, and designed to better safeguard the material over a lifetime, which makes them a perfect collaborator for Global Stem Cells Group.”
Tissue stem cells, or mesenchymal stem cells, harvested from umbilical cord blood and tissue, can differentiate and generate cells of different tissues, mainly connective or supportive, such as bone, cartilage or muscle. Studies show that they can also participate in the process of tissue regeneration through other mechanisms such as immunosuppression, production factors and cell stimulators present in tissue or a damaged organ. Tissue stem cells also have characteristics that allow them to adapt fairly easily to the action of the immune system of an individual, which is why they are essential for use in allogeneic procedures in which immunologic compatibility is a factor.
Stem cells harvested from adipose tissue can differentiate into bone, cartilage, tendon and muscle, and can strengthen defensive and regenerative function. Adipose stem cells are found in adipose (fat) tissue.
Stem cells harvested from dental pulp can also differentiate into bone, cartilage, tendon and muscle to reinforce their defensive and regenerative function. These cells are found in the teeth.
Over time, the body’s stem cells succumb to environmental insults, a common occurrence in the aging process. Cryopreserving stem cells from young patients at an early stage means preserving their maximum viability.
To learn more, visit the Global Stem Cells Group website, email bnovas(at)regenestem(dot)com, or call ![]() 305-224-1858.
305-224-1858.
About the Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV. https://finance.yahoo.com/quote/mssv/
About VidaCel:
Established in 2004,VidaCel® is the largest cell blood bank in Latin America, known for its cryopreservation of umbilical cord stem cells, adipose tissue stem cells and dental pulp stem cells. VidaCel follows the rules of the National Program for Bone Marrow Donation’s. U.S. entity, which also provides guidelines concerning Cord Blood Banks.
Located in Santiago, Chile, VidaCel is the first and only public access stem cell bank in Chile, and guarantees immediate availability of cells. VidaCel is committed to the advancement of new technologies and treatments, in conjunction with leading universities. VidaCel is accredited by the Ministry of Health and the Public Health Institute, and all processes are certified by the international quality standard ISO-9001.
- Published in Press Releases
Global Stem Cells Group, Inc. to Launch Two New Editions of “Diplomat in Cell Therapy and Tissue Engineering” Post-Graduate Diploma Program
Tuesday, December 22, 2015
Global Stem Cells Group will launch two new versions of its “Diplomat in Cell Therapy and Tissue Engineering” post-graduate program. The program will be offered in Chile in 2016
MIAMI (PRWEB) SEPTEMBER 29, 2015
Stem Cell Training, Inc., a division of the Global Stem Cells Group, has announced plans to launch two new editions of the post-graduate diploma program, “Diplomat in Cell Therapy and Tissue Engineering.” The first of its kind worldwide, the program is designed for physicians and qualified practitioners to bring stem cell therapies into the doctor’s office to treat patients.
The program focuses on advances in cell biology that emerged in the late 20th and early 21st centuries to give rise to stem cell therapies—a new form of medical treatment in which cells and tissues are used as healing elements, not only to supplement or replace deficient cells, but to induce regeneration and restoration of a lost biological order during the development of a disease or injury.
According to Global Stem Cells Group CEO Benito Novas, cell therapy is becoming the foundation of future medicine. For that reason, making stem cell sciences and treatment courses available to physicians and qualified medical practitioners is fundamental to the future of medicine. “Diplomat in Cell Therapy and Tissue Engineering” offers professionals invaluable lessons in the new art of healing, as well as the scientific and practical methodologies concerning this new discipline.
The aim of this postgraduate course is to train high-achieving, academic level professionals in cell therapy and tissue engineering to use in different areas of medicine and dentistry.
Course topics include:
- Somatic cell therapy and tissue engineering and associated bioethical issues.
- Cell Structure and functions, cell and tissue growth, cell differentiation and genetic and epigenetic regulation.
- Interaction of cells with other cells and extracellular matrix, humeral microenvironment.
- Development of cell interactions during embryogenesis, tissue regeneration, tissue repair and cancer.
- Immunology: Humeral and cellular immunity, innate and acquired immunity, interactions, effector function, immune tolerance, trophic and reconstructive function.
- Biology of embryo development, and normal and pathological tissue repair: overview of embryonic and fetal post-natal development, genetic and epigenetic factors that influence growth, embryonic fetal and postnatal development, maintenance and repair process of cells, tissue and organs, and genetic and epigenetic factors that interact and influence these processes.
- Identification of certain cellular therapies that repair or correct defects.
- Method of production, processing and application of biological drugs for Cell Therapy and Tissue Engineering.
- Sources of the cells used in cell therapy. Enforcement of cell techniques. Complex manipulation processes, GMP Standards. Cell processing laboratories. The Biosafety and quality of cellular products: Identity, Purity, Power, Security.
- Cell administration. Implant techniques. Evaluating the results of cell therapy.
- Layout of clinical trials.
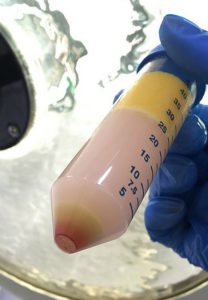 Educational strategies will be taught in theory and in practical hands-on classes, during which students can raise questions and work on problem solving. Practical work will be first carried out on animals; laboratory practices will be taught, followed by demonstration of therapies on humans.
Educational strategies will be taught in theory and in practical hands-on classes, during which students can raise questions and work on problem solving. Practical work will be first carried out on animals; laboratory practices will be taught, followed by demonstration of therapies on humans.
Students will take a written exam on the last day; A score of 7 or better out of 10 is required to pass. Evaluations will be complemented with the development of a thesis, to be graded as pass or fail and must be supported with an oral examination.
To learn more, visit the Stem Cells Training website, the Global Stem Cells Group website, email bnovas(at)regenestem(dot)com, or call ![]() 305-224-1858.
305-224-1858.
About the Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV. https://finance.yahoo.com/quote/mssv/
About Stem Cell Training, Inc.:
Stem Cell Training, Inc. is a multi-disciplinary company offering coursework and training in 35 cities worldwide. Coursework offered focuses on minimally invasive techniques for harvesting stem cells from adipose tissue, bone marrow and platelet-rich plasma. By equipping physicians with these techniques, the goal is to enable them to return to their practices, better able to apply these techniques in patient treatments.
The company’s training courses are designed to make the best use of stem cell technology available to treat various diseases in a manner that is accessible to everyone. Stem Cell Training, Inc.’s mission is to introduce the promising world of cellular medicine to everyone who can benefit from its application, and to provide high quality, effective and efficient training that complies with the highest medical standards to physicians worldwide.
- Published in Press Releases
Global Stem Cells Group in Final Stages of Development of Closed System to Isolate Stem Cells
Tuesday, December 22, 2015
MIAMI (PRWEB) NOVEMBER 30, 2015
Global Stem Cells Group today announced the opening of a new, Good Manufacturing Practice (GMP) 10000 in the Santiago Marriott. The Global Stem Cells Group GMP facility is equipped with the most advanced technologies available, and is operated by a world-class team of qualified medical researchers and practitioners, experienced in administering stem cell protocols using highly manipulated cells.
GMPs are critical for cell therapy success. Understanding and incorporating GMP standards in clinical and pre-clinical research settings establishes a solid foundation on which to build clinical trials and to ensure the safety of biologic products. Good manufacturing practices ensure the safety and efficiency of stem cell products and the patients who receive them. The fundamentals of GMCs are also becoming an important part of the training received by academic researchers in the biotechnology industry, who are at the forefront of developing products for eventual clinical use.

The Global Stem Cells Group GMP 1000 facility will begin receiving international patients in early January 2016.
The GSCG therapeutic center in Santiago—a separate facility from the CMP 1000—will begin seeing international patients as of Dec. 1, 2015 to make its stem cell therapies and facilities available to international patients as well.
For more information, visit the Global Stem Cells Group website, Email bnovas(at)stemcellsgroup(dot)com, or call ![]() 305-560-5337.
305-560-5337.
About the Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions.
With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Groups corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCGs six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV. https://finance.yahoo.com/quote/mssv/
###
- Published in Press Releases