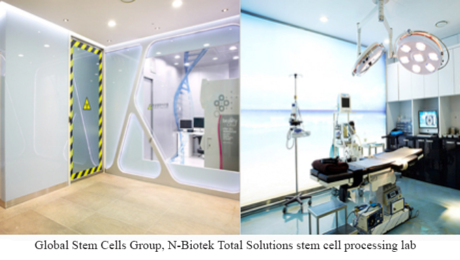
Global Stem Cells Group Announces Permanent Stem Cell Training Center in South Korea
MIAMI, Aug. 30, 2016 – Global Stem Cells Group and its subsidiary Stem Cell Training, Inc., in a collaborative agreement with South Korean biomedical company N-Biotek, announce the establishment of a permanent stem cell training center at the N-Biotek headquarters in Seoul, South Korea. The announcement comes as part of an ambitious expansion effort between GSCG and N-Biotek to provide stem cell training to qualified physicians worldwide.
Stem Cell Training’s two-day intensive “Adipose-derived Stem Cell and Bone Marrow and Platelet Rich Plasma (PRP)” training course covers concepts in cellular medicine, cell viability basics, basic fat and bone marrow stem cell harvesting, isolation and processing procedures, and principles of platelet rich plasma (PRP) processing.
The Korean training center will offer GSCG’s full array of Stem Cell Training courses including:
The “Cell Assisted Fat Transfer” training course is designed for physicians in the aesthetic and cosmetic fields of medicine. The course specifically focuses on procedures to address facial aging caused by volume loss. Course training covers the basics of facial anatomy, facial aging, and the use of fat harvested from the patient’s own body as a filler.
The “Cell Assisted Fat Transfer” training prepares the physician to perform a mini liposuction procedure in order to retrieve fat for grafting in the patient’s anti-aging treatment. It also provides instruction on placement techniques for female and male patients according to age and ethnicity. Fat is a popular filler in the aesthetic medicine field, as the industry experiences an increase in liposuction procedures, an increased interest in facial re-volumization and body, and a growing appreciation for the regenerative potential of adipose-derived mesenchymal stem cells.
The “Diplomat Cell Therapy and Tissue Engineering” course is offered to physicians and qualified practitioners interested in implementing regenerative medicine therapies. This course offers a detailed, hands-on experience in stem cell characterization and laboratory applications; cell protocols including culture, plating, trypsinization, harvesting and cryopreservation; instruction in applying quality control tests including cell count, viability, flow cytometry, endotoxin, mycoplasma and sterility; the ability to perform cGMP functions including clean room maintenance, gowning and environmental monitoring, and new insight and relevant applications of stem cell processing and regulations in a certified facility.
The “Online SVF and Bone Marrow” training course is an online stem cell training course that provides physicians an opportunity to take the clinical training course in a web based format presented as video and voiced over slides. The online course content is consistent with Stem Cell Training’s clinical training course, as it focuses on autologous cellular treatments such as adult stem cells and platelet rich plasma (PRP). Attendees learn how to extract lipoaspirate and bone marrow aspirate, and how to isolate adipose and bone marrow derived stem cells.
In addition, all techniques and materials presented in the training videos are accompanied with detailed protocols.
This course also provides attendees with the tools necessary to implement regulatory and clinical guidelines when setting up a GMP facility; copies of presentations, procedural protocols and all forms associated with a GMP facility; the ability to perform clinical procedures including lipoaspirate and bone marrow isolation; the ability to reintroduce stem cells for various indications, and case books and full protocols for approximately 30 indications.
The two-day online clinical course includes in-clinic procedural videos, recorded didactic lectures, additional information and videos on regenerative medicine, and digital versions of course procedures, protocols and videos.
Stem Cell Training’s experienced and knowledgeable trainers will cover indications, contraindications, patient selection, pre- and post-treatment instructions, and treatment alternatives for autologous facial fat transfer. In addition, participants will learn to analyze anatomy for appropriate areas of facial enhancement; learn various injection techniques to correct temporal hollows, volume loss, tear trough deformity, pre jowl sulcus, malar fat pad, submalar areas, and melomental and nasolabial folds; learn to revent and manage cell-assisted autologous facial fat transfer complications, and understand anesthetic techniques.
To learn more about the stem cells training course center in South Korea, visit the Global Stem Cells Group website, or the Stem Cell Training website, email bnovas@stemcellsgroup.com, or call +1 305 560 5337
About Global Stem Cell Group:
Global Stem Cells Group is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
 About Stem Cell Training, Inc.:
About Stem Cell Training, Inc.:
Stem Cell Training, Inc. is a multi-disciplinary company offering coursework and training in 35 cities worldwide. The coursework offered focuses on minimally invasive techniques for harvesting stem cells from adipose tissue, bone marrow and platelet-rich plasma. By equipping physicians with these techniques, the goal is to enable them to return to their practices, better able to apply these techniques in patient treatments.
About N-Biotek:
Since 1982, N-Biotek has been the leading manufacturer of biomedical and lab equipment worldwide. The Stem Cell Total Solution lab is a culmination of years  of dedication to establish compact and customized products in developing the Handy Lab-a personal lab for medical, scientific and university professionals. N-Biotek is known for its high quality, uniquely designed biomedical products and equipment and competitive pricing. Over the years, the company’s products have earned an array of patents, and all meet international standards including CE, ETL, ISO and GMP. N-Biotek also offers real-time monitoring through IT technology.
of dedication to establish compact and customized products in developing the Handy Lab-a personal lab for medical, scientific and university professionals. N-Biotek is known for its high quality, uniquely designed biomedical products and equipment and competitive pricing. Over the years, the company’s products have earned an array of patents, and all meet international standards including CE, ETL, ISO and GMP. N-Biotek also offers real-time monitoring through IT technology.
Since 2010, N-Biotek has expanded significantly, starting new businesses including a stem cell processing system and biological clean room, GMP consulting, validation services and health care services for foreign consumers in order to maintain its lead in the life science field.
To view this press release live online, click here
###
- Published in Press Releases
Global Stem Cells Group Expects Record Attendance at 3rd Annual International Symposium on Stem Cells and Regenerative Medicine in Buenos Aires
Buenos Aires stem cell symposium scheduled for Sept. 28, 2016.
MIAMI, Aug. 30, 2016 – Global Stem Cells Group, in conjunction with Julio Ferreira, M.D., President of the South American Academy Cosmetic Surgery, will host the 3rd annual Global Stem Cells Group International Symposium on Stem Cells and Regenerative Medicine Sept. 28, 2016. The event, expected to attract a record number of physicians, researchers and regenerative medicine experts from around the world, offers an opportunity for many of the world’s most respected authorities on stem cell and regenerative medicine to showcase advancements in research and therapies on a global level.
Enrique Testart, M.D.
According to Benito Novas, Global Stem Cells Group CEO, the world-class event will showcase the historic advances stem cell medicine has achieved since the first symposium was held just two years ago in 2014.

Joseph Purita, MD
“This year, we will be able to showcase how far stem cell therapies have come since then, and provide some of the most influential leaders that understand the potential of these therapies and have dedicated their careers to making them a reality.”

Sylvina Pastrana, M.D.
To learn more about the 3rd Annual International Symposium on Stem Cells and Regenerative Medicine, visit the Global Stem Cells Group website, email bnovas@stemcellsgroup.com, or call 305-560-5337.
About Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
About Julio Ferreira, M.D.
Julio Ferreira, M.D., President of the South American Academy of Cosmetic Surgery, is director of Clinica Ferreira in Argentina and a professor at the Institute of Biomedical Sciences, University of Sao Paulo, Brazil.
Julio Ferreira, M.D., is an internationally recognized and respected cosmetic surgeon and professor of medicine and aesthetic surgery at the Institute of Biomedical Sciences, University of Sao Paulo, Brazil;
As director of Clinica Ferreira in Argentina, Dr. Ferreira is dedicated to the combination of art and science in aesthetic medicine.
To view this press release live online, click here
###
- Published in Press Releases
Enrique Testart, M.D.
Enrique Testart, M.D., Global Stem Cells Group’s Chief Medical Officer (CMO), is a surgeon specializing in child trauma microsurgery.
Dr. Testart is also a medical entrepreneur and founder of Consortia Innovas S.A. in Santiago, Chile, dedicated to consulting and clinical health management for clinical management firms and research and development-oriented planners in the latest treatments in regenerative medicine as they become available.
A native of Santiago, Chile, Dr. Testart’s medical studies took him all over the world, including orthopedics studies under the direction of Prof. Jean Paul Metaizeau, M.D. in France.
He is in charge of all Global Stem Cells Group divisions and programs in Chile, including patient recruitment through Cellgenic, medical training and certification through Stem Cell Training Inc., and everything related to the sale of equipment disposable through Adimarket.
- Published in Advisory Board Members
Global Stem Cells Group and N-Biotek Collaborate to Offer Turnkey Solutions for Highly Manipulated Stem Cell Culture Expansion Labs
MIAMI, Aug. 23, 2016 – Global Stem Cells Group and its subsidiary Adimarket announce a collaborative agreement with South Korean biomedical and lab equipment manufacturer N-Biotek to offer the Total Solution highly manipulated stem cell culture expansion lab, complete with equipment, training, facilities, stem cell processing center, clean rooms, project plan and more- a total turnkey lab solution for emerging stem cell businesses and industry professionals.
The announcement marks a new chapter in GSCG’s ongoing expansion, and commitment to bring stem cell therapies to

Physicians at work in Total Solution turnkey stem cell lab
Until now, Admarket has been GSCG’s online marketplace for quality regenerative medicine equipment and supplies for physicians and health care professionals, offering basic tools and products to facilitate in-office stem cell procedures. However, through N-Biotek, Adimarket now offers the Total Solution labs and all the accoutrements. From planning to building the facility, to training and consultation, Total Solution users are equipped in every way possible to walk in and begin using their fully-equipped biotechnology lab in short order.

Total Solutions clean room and stem cell lab equipment
The Stem Cell Total Solution is a comprehensive business solution that provides the entire stem cell processing system-from business planning to business procedures, a state-of-the-art cellular processing facility with bio safety clean rooms for stem cell processing, quality control rooms, core technology, cutting-edge equipment, installation, training-everything needed to launch a stem cell business or a enhance a medical research practice in a short period of time.
N-Biotek has been providing its Stem Cell Total Solution labs and consulting services, as well as stem cell processing technology management services, to universities, research centers, physicians and biotechnology enterprises in South Korea, China, Japan and Vietnam for years.
 Highly manipulated stem cells are critical to biotechnology researchers in order to advance both fundamental knowledge of the undifferentiated cells that have the remarkable potential to develop into many different cell types in the body during early life and growth, and to find ways to use them to treat medical and cosmetic conditions non-invasively. Additionally, in many tissues, stem cells serve as a sort of internal repair system, dividing essentially without limit to replenish damaged and dead cells for as long as the person or animal they inhabit is alive.
Highly manipulated stem cells are critical to biotechnology researchers in order to advance both fundamental knowledge of the undifferentiated cells that have the remarkable potential to develop into many different cell types in the body during early life and growth, and to find ways to use them to treat medical and cosmetic conditions non-invasively. Additionally, in many tissues, stem cells serve as a sort of internal repair system, dividing essentially without limit to replenish damaged and dead cells for as long as the person or animal they inhabit is alive.When a stem cell divides, each new cell has the potential to remain a stem cell or become another type of cell with a more specialized function, such as a muscle cell, a red blood cell, or a brain cell, and this allows researchers to manipulate these extraordinary reconstructionist micro-organisms to differentiate into other cell types that can be used to treat disease and injuries affecting parts of the body from which they did not originate. For instance, a stem cell harvested from body fat can be manipulated to take on the functions of a pancreatic cell, a heart cell or a retina cell. Researchers see unlimited potential in their ability to heal wounds, cure diseases, and even prevent birth defects.
 “Non-destructive manipulation of stem cells in a precise environment is key to facilitating discoveries within the biology and medical research communities,” says Benito Novas, CEO of Global Stem Cells Group. “Our collaboration with N-Biotek to deliver the Total Solution for highly manipulated stem cell culture expansion labs to emerging stem cell scientists is the next phase in GSCG’s mission to fulfill our promise of making stem cell-based therapies available to patients everywhere.
“Non-destructive manipulation of stem cells in a precise environment is key to facilitating discoveries within the biology and medical research communities,” says Benito Novas, CEO of Global Stem Cells Group. “Our collaboration with N-Biotek to deliver the Total Solution for highly manipulated stem cell culture expansion labs to emerging stem cell scientists is the next phase in GSCG’s mission to fulfill our promise of making stem cell-based therapies available to patients everywhere.“Stem cell manipulation resulting in cells that possess the characteristics necessary for successful differentiation, transplantation, and engraftment is critical to discovering new methods for treating illness and impairments,” Novas says.
Global Stem Cells Group and N-Biotek provide Total Solution users with everything they need to culture, expand and cryopreserve stem cells right away. Each system comes with manufacture patents and full certification. All products and
 equipment is made to comply with, or exceed standards required for CE Certification.
equipment is made to comply with, or exceed standards required for CE Certification.N-Biotek is the only company that builds the entire stem cell processing system for clients ready to begin a stem cell business, or establish a lab facility to enhance their medical or research practice.
To learn more about the N-Biotek Stem Cell Total Solution, visit the Global Stem Cells Group website at or the AdiMarket website, email bnovas@stemcellsgroup.com, or call 305-560-5337.
About Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
About Adimarket:
Adimarket, Inc., a subsidiary of Global Stem Cells Group, is a cost-competitive online marketplace for quality regenerative medicine equipment and supplies for physicians and health care professionals. Adimarket was founded to provide physicians and other health care professionals the tools they need to practice regenerative medicine in a medical office setting.
Motivated by a firm belief in the positive impact the practice of stem cell medicine can have when dispensed in a doctor’s office, Adimarket provides physicians with the tools they need to provide patients with cutting edge treatments. Adimarket’s experienced customer service representatives provide valuable guidance and advice regarding products relevant to individual practices.
About N-Biotek:
Since 1982, N-Biotek has been the leading manufacturer of biomedical and lab equipment worldwide. The Stem Cell Total Solution lab is a culmination of years of dedication to establish compact and customized products in developing the Handy Lab-a personal lab for medical, scientific and university professionals. N-Biotek is known for its high quality, uniquely designed biomedical products and equipment and competitive pricing. Over the years, the company’s products have earned an array of patents, and all meet international standards including CE, ETL, ISO and GMP. N-Biotek also offers real-time monitoring through IT technology.
Since 2010, N-Biotek has expanded significantly, starting new businesses including a stem cell processing system and biological clean room, GMP consulting, validation services and health care services for foreign consumers in order to maintain its lead in the life science field.
To view this press release live online, please click here
###
- Published in Press Releases
Global Stem Cells Group Announces Stem Cell Training in Madrid
[su_spacer]
Physicians at a recent stem cell training course
MIAMI, Aug. 16, 2016—Global Stem Cells Group and Stem Cell Training, Inc. will host a stem cell training course in Madrid, Dec. 6-7, 2016, in collaboration with Javier Garcia Alonso, MD of Clinica Castellana Norte in Madrid. Garcia Alonso is a specialist in plastic, reconstructive and aesthetic surgery.
The stem cell training course, available to qualified physicians, will focus on stem cell protocols in cosmetic, anti-aging, and aesthetic procedures.
The Adipose and Bone Marrow Stem Cell Training Course was developed for physicians and high-level practitioners to learn the process through a two-day, intensive, hands-on training program to arm participating physicians with clinical protocols and state-of-the-art techniques for isolating and re-integrating adipose- and bone marrow-derived stem cells. When used in aesthetic medicine therapies, patients walk away with more natural-appearing augmentation and enjoy a faster recovery period with little to no downtime.
Garcia Alonso will host and participate in training qualified physicians in stem cell harvesting, isolating and re-integration  techniques and protocols, during which stem cells are harvested from the patient’s own body and redistributed to areas of the body receiving augmentation.
techniques and protocols, during which stem cells are harvested from the patient’s own body and redistributed to areas of the body receiving augmentation.
Stem cell therapy is a promising treatment for facial rejuvenation and soft tissue augmentation because there are no incisional scars or complications associated with foreign materials. Demand for stem cell procedures s high worldwide—these effective procedures that do not involve going under the knife are driving the growth of stem cell protocols in the cosmetic industry, as more patients request new technologies that are less invasive than ever before.
Breast augmentati on, abdominoplasty, facelifts and liposuction continue to feature in the top most requested procedures for many clinics, surgeons say an increasing number of patients are seeking a more natural look with a quicker recovery time, according to Benito Novas, CEO of Global Stem Cells Group. In fact, non-surgical cosmetic procedures have grown exponentially over the past five years, and the trend is expected to continue to rise.
on, abdominoplasty, facelifts and liposuction continue to feature in the top most requested procedures for many clinics, surgeons say an increasing number of patients are seeking a more natural look with a quicker recovery time, according to Benito Novas, CEO of Global Stem Cells Group. In fact, non-surgical cosmetic procedures have grown exponentially over the past five years, and the trend is expected to continue to rise.
Surgeons are also seeing more diver sity when it comes to the type of procedures requested, including buttock enhancements, hair transplantation and anti-aging procedures on hands.
sity when it comes to the type of procedures requested, including buttock enhancements, hair transplantation and anti-aging procedures on hands.
“Patients are demanding procedures that are quick, easy, and provide a fast recovery with minimal downtime,” Novas says. “Stem cells provide the ability to rejuvenate and heal, making them a natural treatment for cosmetic and anti-aging applications.”
The stem cell training course will be offered through Global Stem Cells Group affiliate Stem Cell Training, Inc.
To learn more, visit the Global Stem Cells Group website, or the Stem Cell Training website, email bnovas(at)regenestem(dot)com, or call +1 305 560 5337.
About Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
About Stem Cell Training, Inc.:
Stem Cell Training, Inc. is a multi-disciplinary company offering coursework and training in 35 cities worldwide. The coursework offered focuses on minimally invasive techniques for harvesting stem cells from adipose tissue, bone marrow and platelet-rich plasma. By equipping physicians with these techniques, the goal is to enable them to return to their practices, better able to apply these techniques in patient treatments.
About Clinica Castellana Norte:
Clinica Castellana Norte in Madrid of
fers a comprehensive range of aesthetic and reconstructive procedures, from dietary advice to state-of-the-art stem cell therapies for cosmetic procedures. Clinica Castellana Norte operates under the highest quality standards, not only in medical and aesthetic treatments offered, but throughout the administrative process, ensuring privacy. Clinica Director Javier Garcia Alonso, M.D., is a member of the Spanish Society of Plastic Reconstructive and Aesthetic Surgery (SECPRE), and a member of the International Society of Plastic, Reconstructive and Aesthetic Surgery (IPRAS).
To view this press release live online, click here
###
- Published in Press Releases
Global Stem Cells Group to Honor Joseph Purita, MD at 3rd Annual International Symposium on Stem Cells and Regenerative Medicine in Buenos Aires
Global Stem Cells Group will honor Joseph Purita, MD at its 3rd Annual International Symposium on Stem Cells and Regenerative Medicine, to be held in Buenos Aires Sept. 28, 2016. Purita, an active contributor to the Miami-based biotech company, has been a key participant in GSCG’s growth since it launched in 2013.
[su_spacer size=”20″]

Joseph Purita, MD

Julio Ferreira, MD
The GSCG Symposium offers an opportunity for many of the world’s most respected authorities on stem cell and regenerative medicine to join Purita and other industry leaders to showcase advancements in research and therapies on a global level.

Duncan Ross, PhD
An interdisciplinary team of stem cell experts will provide a full day of high-level scientific lectures geared to medical professionals. Pioneers and luminaries in stem cell medicine will serve as featured speakers, led by keynote speakers Purita and Duncan Ross, PhD Ross, a GSCG Advisory Board faculty member, is a molecular biologist, immunologist and researcher, and the founder of Kimera Labs.
According to Benito Novas, Global Stem Cells Group CEO, the world-class event will showcase the historic advances in stem cell medicine achieved since the first symposium was held just two years ago in 2014.
“We’ve come so far since 2014, and this year’s symposium will highlight the strides Global Stem Cells Group has made in these two years,” Novas says. “And it is only fitting that we take the opportunity to acknowledge Dr. Purita’s contributions to our growth.
“This year, we will showcase how far stem cell therapies have come, and provide some of the most influential leaders like Dr. Purita, who understand the potential of these therapies and have dedicated their careers to making them a reality.”
Since 2014, Global Stem Cells Group has joined forces with some of the most prestigious regenerative medicine practitioners in South America as it focuses on growing its services throughout the global community. Stem cell therapies continue to revolutionize the anti-aging aesthetics industry while offering new hope for sufferers of serious chronic debilitating diseases.
To learn more about the 3rd Annual International Symposium on Stem Cells and Regenerative Medicine, visit the Global Stem Cells Group website, email bnovas(at)regenestem(dot)com, or call 305-560-5337.
About Global Stem Cells Group:
 Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
About Joseph Purita, MD
Joseph Purita, MD, a pioneer in the use of Stem Cell and PRP therapy for orthopedic conditions, graduated from Georgetown University Medical School and served his surgical internship at the University of Florida Medical Center. Following completion of a residency in orthopedic surgery at University of Miami-Jackson Memorial Hospital, where he served as chief administrative resident, Dr. Purita joined the Boca Raton Orthopaedic Group in 1981.
Purita is a Fellow, American Academy of Orthopedic Surgeons; Fellow, American College of Surgeons; member, American Medical Association; member, Southern Medical Association; member, Palm Beach Medical Society; member, Broward County Medical Society; member, Palm Beach Orthopedic Society, and member, Florida Medical Association. His certifications include the American Board of Orthopedic Surgery; American College of Orthopaedic Surgery; American Board of Pain Management, and the American Board of Regenerative Medicine.
Purita is an instructor and proctor of surgeons in the use of lasers in arthroscopic and orthopedic surgery at a variety of area hospitals, and heads GSCG’s Scientific Advisory Board.
To view this press release live online, click here
###
- Published in Press Releases
Julio Ferreira, M.D.
Julio Ferreira, M.D.
Global Stem Cells Group Advisory Board member
Julio Ferreira, M.D., is an internationally recognized and respected cosmetic surgeon and professor of medicine and aesthetic surgery at the Institute of Biomedical Sciences, University of Sao Paulo, Brazil;
As director of Clinica Ferreira in Argentina, Dr. Ferreira is dedicated to the combination of art and science in aesthetic medicine.
Dr. Ferreira serves as president of the South American Academy of Cosmetic Surgery and expert examiner at the International Board of Cosmetic Surgery. He also serves on the International Editorial Advisory Board of the American Journal of Cosmetic Surgery; former President of the International Academy of Cosmetic Surgery 2005/2007; a member and examiner, International Board of Cosmetic Surgery; Corresponding Fellow of the American Academy of Cosmetic Surgery; Honorary Member of the Spanish Society of Cosmetic Medicine and Surgery; Honorary Member of the Eurorusa Confederation of Societies of Aesthetic Plastic Surgery; Honorary Member of the Bulgarian Society of Cosmetic Surgery; Honorary Member of the Chilean Society of Cosmetic Surgery and Lipoplasty; Honorary Member of the Italian Society of Aesthetic Surgery, and Honorary Member of the French Society of Aesthetic Surgery.
- Published in Advisory Board Members
Joseph Purita, M.D.
Joseph Purita, M.D.
Global Stem Cells Group Advisory Board member
Joseph Purita, M.D., is a world-renowned orthopedic surgeon and stem cell pioneer, using cutting edge technology in regenerative medicine in conjunction with stem cell platelet rich plasma (PRP) therapy to treat orthopedic injuries and relieve pain. He is also a pioneer in the use of the laser in orthopedic surgery.
Named a U.S. News and World Report Top Doctor in 2012, Dr. Purita, a renown orthopedic and arthroscopic surgeon, heads Global Stem Cells Group’s Scientific Advisory Board. A pioneer in the use of stem cell and PRP therapy for orthopedic conditions, Dr. Purita has practiced with the Boca Raton Orthopaedic Group in Boca Raton, Florida since 1981. In 2012, Purita gained international attention when he treated New York Yankees pitcher Bartolo Colon’s ligament damage with stem cells, restoring the athlete’s injured shoulder and career. Purita has since treated an array of professional athletes with career-threatening injuries.
Dr. Purita is the director of the Institute of Regenerative and Molecular Orthopedics in Boca Raton, Florida, specializing in the use of stem cells and PRP injections for use in sports medicine and other musculoskeletal conditions. The Institute has treated some of the most prominent professional athletes from all major sports in both the U.S.. and abroad.
He is an instructor and proctor of surgeons in the use of lasers in arthroscopic and orthopedic surgery at a variety of area hospitals,
Dr. Purita is a Fellow, American Academy of Orthopedic Surgeons; Fellow, American College of Surgeons; member, American Medical Association; member, Southern Medical Association; member, Palm Beach Medical Society; member, Broward County Medical Society; member, Palm Beach Orthopedic Society, and member, Florida Medical Association. His certifications include the American Board of Orthopedic Surgery; American College of Orthopaedic Surgery; American Board of Pain Management, and the American Board of Regenerative Medicine.
He is Is Board Certified By The Following Organizations:
• American Board of Orthopedic Surgery
• American College of Orthopaedic Surgery
• American Board of Pain Management
Dr. Purita is a popular speaker at regenerative and orthopedic conferences worldwide.
- Published in Advisory Board Members
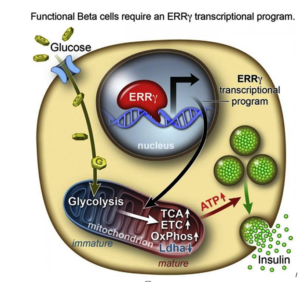
Insulin-producing Stem Cells Grown in the Lab Mark a New Era in Stem Cell Therapies for Diabetes
Introduction to Insulin-Producing Pancreatic Beta Cells
A new discovery by researchers on how to activate lab-grown beta cells to mature into functioning cells that produce and release insulin in response to glucose takes a significant step toward a cell therapy treatment for diabetes. Difficulties in manipulating beta cells derived from human stem cells to mature beyond the precursor stage into fully functioning insulin releasers have been an ongoing challenge for researchers.
Breakthrough by Salk Institute Researchers
However, researchers from the Salk Institute for Biological Studies and a team of researchers have achieved this goal with lab-grown beta cells by activating a protein called estrogen-related receptor γ (ERRγ). Their study findings were recently published in the journal Cell Metabolism.
The Role of Human Pluripotent Stem Cells (hPSCs)
Ronald Evans, senior author of the study titled, “ERRγ Is Required for the Metabolic Maturation of Therapeutically Functional Glucose-Responsive β Cells,” explains the self-renewing capacity of human pluripotent stem cells (hPSCs) and their ability to differentiate into most cell types—from neurons to skin cells, to muscle cells, and insulin-producing pancreatic beta cells—has inspired many research teams to find ways to make glucose-responsive beta cells in the lab. Evans and his research team discovered the answer to the insulin-releasing cell conundrum, and summed it up thusly: “In a dish, with this one switch, it’s possible to produce a functional human beta cell that’s responding almost as well as the natural thing.”
Challenges in Lab-Grown Pancreatic Beta Cells
Evans, a molecular biologist at the Salk Institute, says that to create the different types of cells in the lab, researchers coax the pluripotent stem cells (hPSCs) down the various branching paths that fetal cells normally travel in order to differentiate into the various cell types. However, he explains there are many developmental points in this process, and in the case of lab-grown pancreatic beta cells, research kept getting stuck at an early stage.
Role of ERRγ in Adult Beta Cells
In order to determine what might trigger the next step in getting the cells to mature, the researchers compared transcriptomes of adult and fetal beta cells. The transcriptome contains, among other things, the full catalog of molecules that switch genes on and off in the genome, which led them to discover that the nuclear receptor protein ERRγ was more abundant in adult beta cells. The team was already familiar with the protein’s role in muscle cells and had studied its ability to enhance endurance running. Evans says that in muscles, the protein promotes greater growth of mitochondria—the power generators inside cells that accelerate the burning of sugars and fats to make energy.
Surprising Findings on Beta Cells
“It was a little bit of a surprise to see that beta cells produce a high level of this regulator,” Evans says. “But beta cells have to release massive amounts of insulin quickly to control sugar levels. It’s a very energy-intensive process.” The research team then decided to run some tests to look more closely at what role ERRγ might play in insulin-producing beta cells.
A New Era in Creating Functional, Insulin-Producing Beta Cells

After they genetically engineered a deficiency of ERRγ in mice, the researchers found the animals’ beta cells did not produce insulin in response to spikes in blood sugar. Next, they tried to get beta cells made from hPSCs to produce more ERRγ, and it worked! The cells in culture began to respond to glucose and release insulin. Finally, the team transplanted the lab-grown insulin-producing beta cells into diabetic mice and found that from day one, the cells produced insulin in response to glucose spikes in the animals’ blood.
Future Implications and Research
Evans and the research team were justifiably excited by the results. It appears that just switching on the ERRγ protein is sufficient to get the lab-grown beta cells to mature and produce insulin in response to glucose – both in cultures and in live animals. Speculating on the implications of their findings, Evans suggests that when a fetus is developing, because it gets a steady supply of glucose from the mother, it does not need to produce insulin to regulate its blood sugar, so the switch is inactive. But, when the baby is born and takes its first breath and takes in oxygen, this activates the switch. Previous lab attempts to produce beta cells got stuck at the fetal stage. The Salk Institute researchers discovered how to take it to the adult stage, using the same protein that is switched on in nature.
Conclusion
“I believe this work transitions us to a new era in creating functional beta cells at will,” Evans says. He and his research team now plan to examine how the switch might work in more complex models of diabetes treatments. The Salk Institute study precedes another study reported by Medical News Today in which researchers generated mini-stomachs that produce insulin when transplanted into mice.
- Published in Corporate News / Blog
Global Stem Cells Group Subsidiary Adimarket Announces Progenikine™ SVF Closed System Now Available to Purchase Online
Global Stem Cells Group subsidiary Adimarket announces that Progenikine™, is now available to purchase through the Adimarket website. Progenikine is the new SVF closed system kit utilizing EmCyte technology and containing all the elements necessary to process adipose tissue and obtain stromal vascular fraction in a sterile environment for stem cell therapies.
MIAMI, July 30, 2016–Adimarket, a subsidiary of Global Stem Cells Group, Inc., has announced that Progenikine™, the new and approved SVF closed system kit using EmCyte technology, is now available to purchase online through the Adimarket website. The Progenikine kit contains all the elements necessary to process adipose tissue and obtain stromal vascular fraction (SVF) in a closed environment.
A growing number of physicians are switching to the Progenikine kit system, as it provides the perfect preparation for virtually all clinical applications.
Built with EmCyte Technology, the kit has been independently reviewed and proven in various critical performance points that make a difference in patient outcomes. The Progenikine system allows entire procedure s to be performed in a sterile closed system. Currently, the Progenikine kit is being used in topical procedures such as intra-articular injection for osteoarthritis of the knee and hip, cosmetic surgery and acne scarring, dermal injection, stem cell enriched fat transfer, wounds, chronic ulcers, and other chronic conditions.
s to be performed in a sterile closed system. Currently, the Progenikine kit is being used in topical procedures such as intra-articular injection for osteoarthritis of the knee and hip, cosmetic surgery and acne scarring, dermal injection, stem cell enriched fat transfer, wounds, chronic ulcers, and other chronic conditions.
Adipose derived stem cells (ASCs) are used by physicians for a variety of indications. Most commonly, ASCs are isolated at the point of care from lipoaspirate (derived from liposuction) tissue as the stromal vascular fraction (SVF), harvested from the patient and immediately administered to the patient as an injection, or used to enrich fat grafts. Isolation of ASCs from adipose tissue is a relatively simple process performed routinely in cell biology laboratories, but isolation at the point of care for immediate clinical administration requires special methodology to prevent contamination, ensure integrity of the clinical procedure, and comply with regulatory requirements.
Developed  in conjunction with Patrick Pennie, EmCyte CEO, Progenikine fuses elements from EmCyte systems with the Global Stem Cells Group SVF protocols. The kit can provide a low cost, rapid and simple alternative to traditional methods of isolating ASCs, particularly when smaller quantities are needed.
in conjunction with Patrick Pennie, EmCyte CEO, Progenikine fuses elements from EmCyte systems with the Global Stem Cells Group SVF protocols. The kit can provide a low cost, rapid and simple alternative to traditional methods of isolating ASCs, particularly when smaller quantities are needed.
To learn more about the Progenikine kit, visit the Adimarket website, email bnovas(at)stemcellsgroup(dot)com, or call 305-560-5337.
About Global Stem Cells Group:
Global Stem Cells Group is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions.
With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cel ls Groups corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCGs six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
ls Groups corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCGs six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About Adimarket:
Adimarket, Inc., a division of the Global Stem Cells Group, is a cost-competitive online marketplace for quality regenerative medicine equipment and supplies for physicians and health care professionals.
Adimarket was founded to provide physicians and other health care professionals the tools they need to practice regenerative medicine in a medical office setting. Motivated by a firm belief in the impact the practice of stem cell medicine can have when dispensed in a doctor’s office, Adimarket provides physicians with the tools they need to provide patients with cutting edge treatments.
Adimarket’s experienced customer service representatives provide valuable guidance and advice regarding products relevant to individual practices.
About EmCyte:
 Fort Myers, Florida-based EmCyte Corporation is a leader in autologous cellular biologics with the GenesisCS Component Concentrating Systems. These systems provide patients with the best opportunity for rapid recovery and provide practitioners with the most advanced clinical point of care experience. EmCyte systems are developed to meet every clinical requirement, giving the physician better clinical choices. EmCyte devices have been independently reviewed and show to produce buffycoat concentrations of 6x to greater than 10x baseline in 7mLs, with yields ranging from 70 percent to greater than 90 percent.
Fort Myers, Florida-based EmCyte Corporation is a leader in autologous cellular biologics with the GenesisCS Component Concentrating Systems. These systems provide patients with the best opportunity for rapid recovery and provide practitioners with the most advanced clinical point of care experience. EmCyte systems are developed to meet every clinical requirement, giving the physician better clinical choices. EmCyte devices have been independently reviewed and show to produce buffycoat concentrations of 6x to greater than 10x baseline in 7mLs, with yields ranging from 70 percent to greater than 90 percent.
EmCyte technology allows for the safe extraction of concentrated platelets and other regenerative cell types from the patient’s own blood. These cells are then re-suspended in a small volume of the patient’s blood plasma and then applied to the treatment site.
###
- Published in Press Releases