International Society for Stem Cell Application (ISSCA) and Marmara University Launch Stem Cell and Regenerative Medicine Fellowship Program in Turkey
Participants in the program will have access to over 200 hours of valuable online content and hands-on laboratory practice in the state-of-the-art facilities at Marmara University and StemBio. Generally, the program will cover a series of topics related to the general principles of regenerative medicine, including:
- Stem cell biology;
- Evidence-based treatments; and
- Tissue engineering.
Hear from Certified and Established Industry Leaders
The Stem Cell and Regenerative Medicine Fellowship Program aims to provide attendees with the chance to meet, interact, and learn from ISSCA-certified doctors, scientists, experts, and Marmara University professors with in-depth expertise in the field. There will be seven days of in-person training and a comprehensive digital course offering practical experience in stem cell classifications and laboratory applications, with a high emphasis on Good Manufacturing Practice (GMP) guidelines.
Learn About the Best Stem-Cell Application Practices and Procedures in Clinical and Manufacturing Operations
By attending the program, participants should be able to gain valuable answers to the “how to” questions related to the practical applications of stem cell biology and regenerative medicine in research laboratories and manufacturing facilities. Attendees can expect to learn the following:
- How to culture stem cells;
- How to meet quality control standards;
- How to comply with cGMP functions;
- How to operate within the recommended clinical procedures;
- How to apply and implement the regulations for stem cell processing in a certified manufacturing facility; and
- How to apply clinical guidelines and regulatory policies when developing a GMP facility
Besides, the seven-day fellowship program will also offer participants resources to bolster their stem cell operations, including copies of presentations, procedural guidelines, and all forms and documentation related to a GMP facility.
Moreover, the Stem Cell and Regenerative Medicine Fellowship Program will cover a range of clinical procedures, including:
- Tissue processing;
- Isolation of mesenchymal stem cells;
- Culture, Expansion and cryopreservation; and
- Clinical Application of stem cells for wide-ranging indications: participants will get case books and complete protocols for about 30 indications.
Why Attend the Stem Cell and Regenerative Medicine Fellowship Program?
The program provides a learning experience for medical professionals and scientists who’d love to further their knowledge and skills in stem cells and regenerative medicine. Attendees will get the opportunity to learn from the top professionals in the field and gain practical hands-on experience in world-class laboratory facilities.
Here’s what Global Stem Cells Global’s CEO, Benito Novas, had to say: “We are delighted to hold the Stem Cells and Regenerative Medicine Fellowship Program, which is designed to provide participants with the opportunity to gain and further their knowledge in stem cell biology and tissue engineering. In addition, ISSCA’s partnership with Marmara University will ensure attendees have access to professors with deep knowledge in the field and practical training in stem cell and regenerative medicine.”
For more information on the Stem Cell and Regenerative Fellowship Program, visit the ISSCA website or contact Global Stem Cells Group.
About Marmara University
Marmara University is a leading research university nestled in Istanbul, Turkey. It’s a distinguished academic institution with high authority in scientific research and academic excellence, specifically in medicine. The university provides undergraduate, graduate, and doctorate degrees, as well as certificate programs. Marmara University also boasts state-of-the-art facilities and dedicated faculty that chronicle its dedication to giving scholars world-class learning experiences and contributing to the advancement of knowledge in wide-ranging fields.
About ISSCA:
The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians who aspire to treat diseases and lessen human suffering through advances in science, technology, and the practice of regenerative medicine. ISSCA serves its members through advancements made in the specialty of regenerative medicine.
The mission of the International Stem Cell Certification Agency (ISSCA) is to establish itself as a global leader in regenerative medicine certification, education, research, and training.
ISSCA provides certification training in cities worldwide because it recognizes the importance of standards and certifications in regenerative medicine as a medical specialty. To help more people, both locally and globally, as the demand for more doctors interested in and comfortable with regenerative medicine surges. ISSCA’s mission is to advance quality and uniformity in regenerative medicine worldwide.
About Global Stem Cells Group:
The Global Stem Cell Group is a family of several companies focused on stem cell medicine and research. The company uses its network to bring leadership in regenerative medicine training, research, and patient applications.
GSCG’s mission is to allow physicians to present the benefits of stem cell medicine to patients worldwide. The company also partners with policymakers, educators, and regulators to promote regenerative medicine.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV. https://finance.yahoo.com/quote/mssv/
To learn more about Global Stem Cells Group, Inc.’s companies visit our website www.stemcellsgroup.com or call +1 305 560 5331
Safe Harbor Statement:
Statements in this news release may be “forward-looking statements”. Forward-looking statements include, but are not limited to, statements that express our intentions, beliefs, expectations, strategies, predictions, or any other information relating to our future activities or other future events or conditions. These statements are based on current expectations, estimates, and projections about our business based partly on assumptions made by management. These statements are not guarantees of future performance and involve risks, uncertainties, and assumptions that are difficult to predict. Therefore, actual outcomes and results may and are likely to differ materially from what is expressed or forecasted in forward-looking statements due to numerous factors. Any forward-looking statements speak only as of the date of this news release, and The Global Stem Cells Group undertakes no obligation to update any forward-looking statement to reflect events or circumstances after the date of this news release. This press release does not constitute a public offer of any securities for sale. Any securities offered privately will not be or have not been registered under the Act and may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements.
- Published in Press Releases
Stem Cell Center Network in Lisbon Portugal
The Stem Cell Center Network ( a Division of Global Stem Cells Group ) is an international network of regenerative medicine practitioners . Dedicated to promoting the research and development of the field of regenerative medicine, and strives to bring patients cutting-edge treatments that make use of the ever-growing list of benefits that regenerative medicine carry. This week, in a part of their effort to expand their foothold in medical communities around the world, the Stem Cell Center Network has announced the opening of a new training facility in Lisbon, Portugal.
Over the years, the Stem Cell Center Network has sought out to aggressively expand and roll out new membership opportunities, programs, events, and more– an effort that has been redoubled in the last five years. As a result, we have added members that practice regenerative medicine under our banner in over twenty five countries spread across five continents.
The new Portuguese center will be located in the clinic of Dr. Hugo Madeiras, a highly-accredited physician and scientist who will preside over the facility as the Global Stem Cells Group chief representative in Portugal. As the CEO and founder of the Clinic of Advanced Implantology, he focuses exclusively on implantology, esthetics, and digital workflows, and collaborates with over twenty doctors and seventy resources in total. He is also the clinical collaborator for the Straumann Group, a faculty which includes product testing, lecturing, and the creation and delivery of educational content.
Dr. Madeiras graduated from the Instituto Superior de Ciências da Saúde Egas Moniz in Medicine in 2007, and with a Master in Oral Rehabilitation from CESPU in 2009. With his over ten year of experience in the field, he acts as a speaker at several national and international congresses that go over developments and new treatment protocols in the field of dentistry.
“This new center will help in bringing regenerative medicine to the people of Lisbon. It will treat patients with a wide variety of different diseases, but with a special emphasis on Aesthetic Medicine,” Said Benito Novas CEO of the Global Stem Cells Group about the new Stem Cell Center Network partnership, “This new facility will be at the top of the line, a place where Portuguese doctors can come to learn about regenerative medicine protocols, and the advancements that have been made in the field. I am incredibly excited to be working in partnership with Global Stem Cells Group , and look forward to many years as a program leader here in Portugal,”
Stem Cell Center Network plans for the clinic to open in September 2020– and with that date looming closer and closer with each passing week, a date has also been agreed upon for the clinic’s inaugural training– indeed, it will be a place for doctors around the country to convene and learn, share, and research the complexities and applications of regenerative medicine. Barring any extenuating circumstances, the next first Regenerative Medicine Certification Training in the Portuguese clinic will take place on September 25 & 26th, 2020– just weeks after its formal opening. With only 10 spots available, applicants are encouraged to sign up soon for the hands-on training course.
To sign up today, visit: https://issca.us
About Global Stem Cells Group
Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations, each focused on furthering scientific and technological advancements to lead cutting-edge stem cell development, treatments, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop hub for stem cell solutions that adhere to the highest medical standards.
- Published in Press Releases
Platelet-Rich Plasma Stays Quietly Popular Despite Neglect
Fact: PRP Treatments Are Highly In-Demand
According to research, PRP treatments are one of the most in-demand treatments available in healthcare. This is impressive considering the following challenges PRP faces:
- Lack of Support from the Medical Industry: PRP is not backed by big pharma, meaning no extensive research or marketing is funded.
- Absence of Lobbying by Medical Associations: There are no medical associations working to increase awareness of PRP.
- No Insurance Reimbursements: Insurance companies do not reimburse PRP treatments, making it difficult to get patients to pay for a treatment that is relatively “unproven.”
- Rising Costs: In 2006, a PRP treatment cost $450. Today, it costs $800, with the cheapest being $650.
Despite these obstacles, the demand for PRP treatments remains robust.
The Future of PRP
We believe the best of PRP is yet to come. A breakthrough study could propel PRP into mainstream hospitals and clinics. The most significant growth in PRP is currently happening in Asia, rooted in fundamental healing theory.
The Healing Power of PRP
The growth of PRP can be attributed to its fundamental healing properties:
- Increased Platelets: More platelets mean more growth factors and cytokines, leading to enhanced healing.
- Natural Healing Mechanism: Our body’s natural healing mechanism operates with 150,000/ul-350,000/ul platelets in blood. Using PRP amplifies this number by 3X to 5X, translating to better healing.
Platelet-Rich Plasma Trends
PRP can be used to promote the healing of injured tendons, ligaments, muscles, and joints, and is applied to various musculoskeletal problems. Regular studies test its effectiveness. One landmark study involved double-blind randomized controlled trials to see the effect of PRP on patients with chronic low back pain caused by torn discs. The study found that 60% of the patients felt significant improvements, with some even being cured.
Platelet-Rich Plasma Variants
There are several types of PRP variants:
- Plasma Rich in Growth Factors (PRGF)
- Plasma Rich in Platelets and Growth Factors (PRPGF)
- Platelet-Rich Plasma (PRP)
- Platelet Poor Plasma (PPP)
- Plasma Rich in Platelets and Rich in Leukocytes (LR-PRP)
- Plasma Rich in Platelets and Poor in Leukocytes (LP-PRP)
- Platelet-Rich Fibrin Matrix (PRFM)
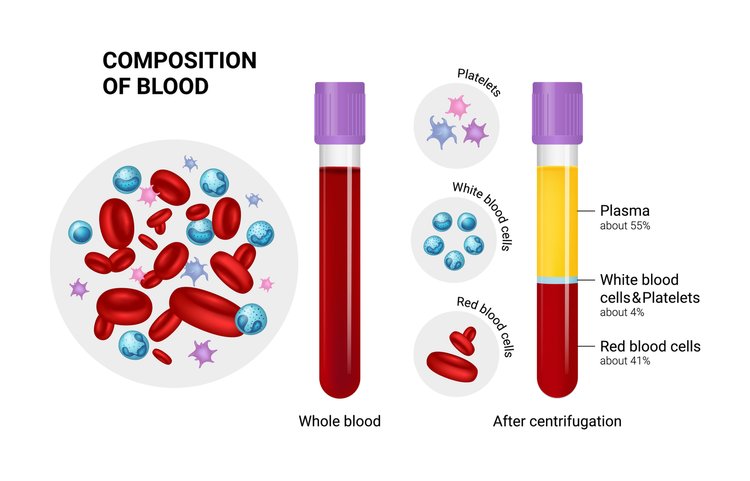
All of them involve plasmapheresis—the two-stage centrifugation process to separate platelets from blood. The industry has yet to standardize which variant to use, but we believe the confusion will clear up in 3-5 years.
Bio-Factors at Play in PRP
Regardless of the PRP variant used, the following bio-factors are at play:
- Growth Factors: TGF-B, PDGF, IGF-I,II, FGF, EGF, VEGF, ECGF
- Adhesive Proteins: Fibrinogen, Fibronectin, Vitronectin, Thrombospondin-1
- Clotting & Anti-Clotting Factors: Proteins, Antithrombin, Plasminogen, Proteases, Antiproteases
How Platelet-Rich Plasma Actually Works
PRP is commonly used for wound healing and pain management because platelets aid coagulation, act as a biological glue, and support stem or primary cell migration. They also help restore hyaluronic acid, accelerate collagen synthesis, and increase cartilage matrix.
Platelets delivered in a clot can immediately act as a scaffold to enable the healing process. 95% of bio-active proteins are released within 1 hour of injecting PRP, and platelets continue to release growth factors for 7-10 days. Thus, it’s recommended to re-inject PRP every 7 days.
Why Patients Choose PRP Despite the Cost
Patients are willing to pay out of pocket for PRP treatments despite insurance companies not covering it because:
- Effectiveness: The treatment works.
- Natural and Side-Effect-Free: There is nothing else as natural and side-effect-free as PRP.
PRP for Osteoarthritis
Consider osteoarthritis, which affects 27 million Americans. When doctors started doing PRP treatments for their osteoarthritis patients, a large majority experienced no further cartilage loss. This suggests PRP should be the default first-line treatment for osteoarthritis across the country.
PRP for Hair Loss and Cosmetic Applications
PRP also has significant potential in treating hair loss and cosmetic facial applications. Studies have shown reduced hair loss and increased hair density, with patients expressing high satisfaction levels.
The Growing PRP Market
The PRP market is expected to hit $126 million in 2016, a 180% increase over the 2009 figure of $45 million. For osteoarthritis alone, if all 27 million Americans received 1 PRP shot a year at a conservative $400 per treatment, it would be a $10 billion market.
PRP for Tennis Elbow
PRP is also known to work well for Tennis Elbow, which affects 1% to 3% of the overall population and up to 50% of tennis players.
Insurance Coverage for PRP
Getting PRP covered by insurance could significantly expand the market and help heal millions of patients more naturally and effectively. It could also save insurance companies money by reducing the need for more expensive interventions like surgery.
Challenges and the Future of PRP
The vast scope of PRP treatment calls for urgent structure and guidelines. There are some 20+ conditions where researchers have found it helpful. Proving its efficiency in all these areas is a daunting task, but we will get there with enough funding and standardized procedures.
Conclusion
Despite being classified as “unproven,” PRP has vast potential. With the right number of platelets, platelet activation, and cytokine release, PRP can consistently deliver positive outcomes. Although there is still uncertainty over the number of injections, timing, and delivery method, widespread adoption will eventually lead to a more structured approach.
Let’s hope the first glimpses of this structure will arrive soon. In 2015, the world saw approximately 1 million knee arthroplasties for osteoarthritis, costing $25 billion. How many of these patients could have benefited from PRP early on?
- Published in Corporate News / Blog
Platelet-Rich Plasma For Melasma — Will It Fade Forever?
For most women, a tiny pimple on the face is enough to ruin their day. Or week. Even the slightest imperfection that may have a 1% chance of getting noticed by others will freak them out. For these women, Melasma is their darkest nightmare. It’s a pretty common issue, a result of exposure to the sun, that causes brown patches on the face. Permanent patches, I should add.
If you’re suffering from Melasma, the road to “recovery” usually looks like this:
- You hope that it’ll fade away.
- Your friend suggests you try apple cider vinegar and lemon juice treatment.
- Slightly disappointed.
- You visit a dermatologist who’ll prescribe a bleaching cream (hydroquinone or similar).
- Full-on disappointment.
- You Google the hell out of the topic.
- Overwhelm.
- Concealers and makeup become your best friend.
At this point, no one can convince you there is a treatment for getting rid of melasma. Trying more and more treatments only runs the risk of making the condition worse. So what would you do?
What About Platelet-Rich Plasma For Melasma?
According to recent Turkish and Malaysian studies, Platelet-Rich Plasma is showing great promise for melasma. The one good thing about PRP for Melasma is the fact that PRP won’t make the condition worse unlike IPL, Fraxel, or other treatments. So that’s one of the treatments you can confidently try without worry. It’s like getting a natural facial treatment that has a whole lot of potential benefits even if it didn’t help cure melasma.
PRP injections work by supplying growth factors to reduce the pigmentation. And being an independent treatment with no downtime, it can be done in conjunction with conventional treatments for melasma to add and enhance the effects. There are more than 30 bioactive substances in Platelet-Rich Plasma that have separate roles like increasing skin volume and adding new blood vessels to name a few.
Platelet-Rich Plasma with Microneedling
This is the most common combination for Platelet-Rich Plasma therapy. Here’s a video of Dr. Michael Somenek performing a PRP injection on a patient of his immediately after microneedling. The combination is known to have produced results for a lot of varieties of skin pigmentation issues that it’d not be wise for anyone to ignore it for melasma, especially when creams and peels didn’t help. More important is PRP’s ability to stimulate collagen production in the area so it tightens the pores and makes your skin glowing.
Why Platelet-Rich Plasma?
PRP is primarily a healing vehicle. It needs to be injected into the membrane below the skin. The way it works is by supplying the underlying skin membrane with collagen and tenascin stimulated by the transforming growth factors in PRP. These growth factors also promote the formation of new blood vessels that in some cases results in the disappearance of spider veins.
The released growth factors (mainly platelet-derived growth factor (PDGF), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and transforming growth factor-beta (TGF-ß)) can stimulate the proliferation of fibroblasts and epidermal cells, and collagen synthesis. In addition, the transforming growth factor-beta (TGF-ß) has been proven to inhibit melanogenesis — or reverse skin pigmentation — the exact opposite effect of exposure to UV-B radiation.
Typically, patients see excellent results with 2-3 PRP injections in the first 3 months. And clinical studies have shown that it will maintain after 6 months. However, Melasma is known to recur even after successful treatments. So you must take precautions against it by using sunscreen with broad-spectrum protection and an SPF of 30 or higher. And avoid skincare products that are harsh as they can exacerbate melasma.
- Published in Corporate News / Blog
tem Cell Platelet-Rich Plasma: The Best Regenerative Therapy?
To understand why stem cell platelet-rich plasma or co-transplantation of adipose-derived mesenchymal stem cells (ADSC) and PRP is such a remarkable idea in regenerative medicine, let’s spend a little time looking at the mechanics of PRP.
Platelet-Rich Plasma’s Role as Repairmen
The one thing that makes Platelet-Rich Plasma a hero in several fields (if not all) of medicine is the fact that the diverse growth factors in it are able to stimulate stem cell proliferation and cell differentiation (the factors that determine effective tissue regeneration and healing) on any part of the body. These growth factors are abundant in the blood and act as the natural repairmen of tissues. In the perfect scenario, there’s plenty of blood flow to every part of the body, and these “repairmen” are always on-call to address any healing needs that may arise. However, if the injured area has a poor blood supply — especially areas that constantly move like tendons, ligaments, and joints — demand for these repairmen can outgrow supply. Meaning, healing (or regeneration of tissues) is put on hold till further repairmen are available.
The train of Platelet-Rich Plasma then arrives with enough of these repairmen to warrant the resumption of healing. There’s another part of this picture we haven’t talked about so far: stem cells.
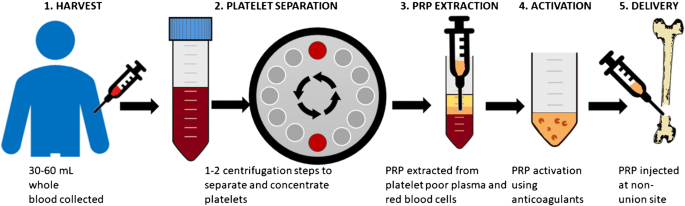
Stem Cells as the Raw Materials for PRP
As far as Platelet-Rich Plasma and its growth factors are concerned, they are mere repairmen. They can’t do the work by themselves. They need the basic raw materials to work with. And that raw material here is stem cells. Stem cells are the ones actually being regenerated to form new tissues for healing.
Supplying Both PRP and Stem Cells for Regeneration
Stem cells are the only raw materials that PRP works with for regeneration. These are like the fundamental building blocks of all other cells. These cells can be guided into becoming specialized cells under the right conditions. In addition, they can also divide themselves to form new stem cells or new specialized cells. So for Platelet-Rich Plasma to work well, it needs to be applied to an area with lots of stem cells like the heart, liver, blood vessels, etc. Incidentally, Platelet-Rich Plasma’s healing properties were first discovered by cardiac surgeons who played with concentrated blood for faster healing of the heart after surgery, and it showed tremendous promise because stem cells are abundant in heart tissues. But what if healing is needed in an area where there are not many stem cells?
With the new developments in stem cell technology, that can be solved too. Because now we can supply the stem cells to areas where there are less like the joints, ligaments, and tendons. For this, scientists usually use “mesenchymal stem cell” or MSCs. These are cells isolated from stroma and can differentiate to form adipocytes, cartilage, bone, tendons, muscle, and skin. The easiest way is to harvest it from adipose tissue or fat that we call Adipose-derived mesenchymal stem cells or ADSC.
The Future of Stem Cell Platelet-Rich Plasma
In regions with hypoxia (poor blood supply) like joints, meniscus tissue, rotator cuff, spinal discs, etc., the supply of platelets (and therefore growth factors) as well as the stem cells are limited. So what if we supplied both the stem cells and Platelet-Rich Plasma for triggering the regeneration process? That’s the question these Japanese scientists answered in their research. Here’s another group of scientists who took on the same challenge.
They used Adipose-derived mesenchymal stem cells (ADSC), which are known for their ease of isolation and extensive differentiation potential. These researchers noted that these stem cells often can’t survive in areas of local hypoxia, oxidative stress, and inflammation – thereby making them ineffective. However, when Platelet-Rich Plasma (or thrombin-activated PRP) is added to ADSC, it kept them alive for prolonged periods and the growth factors in the Platelet-Rich Plasma triggered cell differentiation and proliferation more easily.
Why This Exact Combination Is the Future
Done this way, both Adipose-derived mesenchymal stem cells (ADSC) and Platelet-Rich Plasma are raw materials for healing that’s already available in plenty in almost everyone (there are exceptions of course). That means, for complete healing to take place, this combination treatment, still in its very primitive stage of development, may have the potential to replace expensive synthetic drugs that carry complex unexplained side effects. The procedure takes our body’s natural healing agents — stem cells from body fat and PRP from blood — and then injects them inside the knee or other joints (or other areas where they are insufficient) for regeneration. Isn’t that like the most wonderful thing ever?
Whether it’s a cartilage cell, a bone cell, or a collagen cell for ligaments and tendons that need to be healed, all you need is a same-day procedure by a local, but specialized doctor, using the natural ingredients of the body. I believe this special combo is a huge win for Platelet-Rich Plasma.
The Challenges for Growing Adoption of This Treatment
We know Platelet-Rich Plasma has safe, yet high-speed recovery potential with its multiple growth factors. And it is effective in regenerative healing of cartilage injuries – the toughest injuries to heal – as well as Osteoarthritis. However, the challenges are Platelet Quality. We need to somehow ensure the Platelet-Rich Plasma quality is uniform. Currently, it varies from two to several fold above baseline concentration based on the donor’s physical condition.
Next, we need to identify the exact PRP growth factors that promote ADSC proliferation. Scientists believe growth factors such as basic fibroblast growth factor (bFGF), epidermal growth factor, and platelet-derived growth factor stimulate stem cell proliferation while some growth factors under certain conditions are known to inhibit the process. The percentage of PRP matters too. Scientists have tested 5 percent, 10 percent, 15 percent, and 20 percent Platelet-Rich Plasma in ADSC.
The Only Treatment in Modern Medicine for Cartilage Regeneration
The bottom line is that Stem Cell Platelet-Rich Plasma or ADSC + PRP procedure is the only treatment in modern medicine that has shown cartilage regeneration. So it’s too important to ignore. And it could be one of the greatest advances that science has brought to the millions of people suffering from serious pain in their joints, knee, and spine as well as people suffering from all kinds of tendon diseases and injuries.
To understand why stem cell platelet-rich plasma or co-transplantation of Adipose-derived mesenchymal stem cells and PRP, is such a remarkable idea in regenerative medicine, let’s spend a little time looking at the mechanics of PRP.
- Published in Corporate News / Blog
Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise
Introduction
The clinical demand for bioengineered blood vessels continues to rise, yet current options for vascular conduits remain limited. The synergistic combination of emerging advances in tissue fabrication and stem cell engineering promises new strategies for engineering autologous blood vessels that recapitulate not only the mechanical properties of native vessels but also their biological function. Here, we explore recent bioengineering advances in creating functional blood macro and microvessels, particularly featuring stem cells as a seed source. We also highlight progress in integrating engineered vascular tissues with the host after implantation and the exciting pre-clinical and clinical applications of this technology.
The Clinical Need for Vascular Grafts
Ischemic diseases, such as atherosclerotic cardiovascular disease (CVD), remain one of the leading causes of mortality and morbidity worldwide (GBD 2015 Mortality and Causes of Death Collaborators, 2016; Mozaffarian et al., 2016). These diseases have resulted in an ever-persistent demand for vascular conduits to reconstruct or bypass vascular occlusions and aneurysms. Synthetic grafts for replacing occluded arterial vessels were first introduced in the 1950s following surgical complications associated with harvesting vessels, the frequent shortage of allogeneic grafts, and immunologic rejection of large animal-derived vessels. Despite advances in pharmacology, materials science, and device fabrication, these synthetic vascular grafts have not significantly decreased overall mortality and morbidity (Nugent and Edelman, 2003; Prabhakaran et al., 2017).
Challenges with Synthetic Grafts
Synthetic grafts continue to exhibit a number of shortcomings that have limited their impact. These shortcomings include low patency rates for small diameter vessels (< 6 mm in diameter), a lack of growth potential for the pediatric population necessitating repeated interventions, and susceptibility to infection. Vascular conduits are also needed for clinical situations such as hemodialysis, which involves large volumes of blood that must be withdrawn and circulated back into a patient several times a week for several hours.
The Importance of Microvascularization
In addition to large-scale vessel complications, ischemic diseases also arise at the microvasculature level (< 1 mm in diameter), where replacing upstream arteries would not address the reperfusion needs of downstream tissues (Hausenloy and Yellon, 2013; Krug et al., 1966). Microvascularization has proven to be a critical step during regeneration and wound healing, where the delay of wound perfusion (in diabetic patients, for example) significantly slows down the formation of granulation tissue and can lead to severe infection and ulceration (Baltzis et al., 2014; Brem and Tomic-Canic, 2007; Randeria et al., 2015).
Structural Components of Blood Vessels
To design advanced grafts, it is important to consider the structural components of a blood vessel. Many different blood vessel beds share common structural features. Arteries, veins, and capillaries have a tunica intima comprised of endothelial cells (EC), which regulate coagulation, confer selective permeability, and participate in immune cell trafficking (Herbert and Stainier, 2011; Potente et al., 2011). Arteries and veins are further bound by a second layer, the tunica media, composed of smooth muscle cells (SMC), collagen, elastin, and proteoglycans, conferring strength to the vessel and acting as effectors of vascular tone. Vascular tissue engineering has evolved to generate constructs that incorporate the functionality of these structural layers, withstand physiological stresses inherent to the cardiovascular system, and promote integration in host tissue without mounting immunologic rejection (Chang and Niklason, 2017).
Suitable Cell Sources
A suitable cell source is critical to help impart structural stability and facilitate in vivo integration. Patient-derived autologous cells are one potential source that has garnered interest because of their potential to minimize graft rejection. However, isolating and expanding viable primary cells to a therapeutically relevant scale may be limited, given that patients with advanced arterial disease likely have cells with reduced growth or regenerative potential. With the advancement of stem cell (SC) technology and gene editing tools such as CRISPR, autologous adult and induced pluripotent stem cells (iPSCs) are emerging as promising alternative sources of ECs and perivascular SMCs that can be incorporated into the engineered vasculature (Chan et al., 2017; Wang et al., 2017).
Engineering and Integration of Vascular Tissues
A viable cell source alone is not sufficient for therapeutic efficacy. Although vascular cells can contribute paracrine factors and have regenerative capacity, merely delivering a dispersed mixture of ECs to the host tissue has shown limited success at forming vasculature or integrating with the host vasculature (Chen et al., 2010). Recent tissue engineering efforts have focused on recreating the architecture and function of the vasculature in vitro before implantation, with the hypothesis that pre-vascularized grafts and tissues enhance integration with the host.
Clinical Applications
The first reported successful clinical application of tissue-engineered blood vessels (TEBV) in patients was performed by Shin’oka et al., who implanted a biodegradable construct as a pulmonary conduit in a child with pulmonary atresia and single ventricle anatomy (Shin’oka et al., 2001). The construct was composed of a synthetic polymer mixture of L-lactide and e-caprolactone, reinforced with PGA, and seeded with autologous bone marrow-derived mesenchymal stem cells (BM-MSCs). The authors demonstrated patency and patient survival 7 months post-implant, and expanded their study to a series of 23 implanted TEBVs and 19 tissue patch repairs in pediatric patients (Hibino et al., 2010). They reported no graft-related mortality, and four patients required interventions to relieve stenosis at a mean follow-up of 5.8 years.
The first sheet-based technology to seed cultured autologous cells, developed by L’Heureux et al., induced cultured fibroblast cell sheets over a 10-week maturation period to produce tubules of endogenous ECM over a production time ranging between 6 and 9 months. They dehydrated and provided a living adventitial layer before seeding the constructs with ECs (L’Heureux et al., 2006). Their TEBV, named the Lifeline graft, was implanted in 9 of 10 enrolled patients with end-stage renal disease on hemodialysis and failing access grafts in a clinical trial. Six of the nine surviving patients had patent grafts at 6 months, while the remaining grafts failed due to thrombosis, rejection, and failure (McAllister et al., 2009).
Future Directions
Harnessing the regenerative functions reported in ECs derived from adult stem cells and iPSCs offers the promise of improving TEBV patency. Mcllhenny et al. generated ECs from adipose-derived stromal cells, transfected them with an adenoviral vector carrying the endothelial nitric oxide synthase (eNOS) gene, and seeded the ECs onto decellularized human saphenous vein scaffolds (McIlhenny et al., 2015). They hypothesized that through inhibition of platelet aggregation and adhesion molecule expression, nitric oxide synthesis would prevent thrombotic occlusion in TEBV. Indeed, they reported patency with a non-thrombogenic surface 2 months post-implantation in rabbit aortas. Engineering ECs and SMCs with other regenerative, anti-inflammatory, and anti-thrombotic genes could bridge the functional difference between SC-derived cells and native primary cells.
- Published in Corporate News / Blog
Purest Liver-like Cells to Date Generated from Induced Pluripotent Stem Cells (iPSCs)
Researchers from the Medical University of South Carolina (MUSC) and the University of Pennsylvania have discovered a new methodology for purifying liver cells generated from induced pluripotent stem cells (iPSCs) that could facilitate progress toward an important clinical goal: treating patients with disease-causing liver mutations by transplanting unmutated liver cells derived from their own stem cells.
Background on Liver Stem Cell Research
This new technique follows previous attempts to generate liver-like cells from stem cells, which have yielded heterogeneous cell populations with little similarity to diseased livers in patients.
The Role of Next Generation Genetic Association Studies Program
The National Heart, Lung, and Blood Institute (NHLBI)’s Next Generation Genetic Association Studies Program (Next Gen) was created to bank stem cell lines sourced from patients in genome-wide association studies (GWAS). The goal of the Next Gen Lipid Conditions sub-section – a collaborative effort between Stephen A. Duncan, Ph.D., chair of regenerative medicine at MUSC, and Daniel J. Rader, M.D., and Edward E. Morrisey, Ph.D., both at the University of Pennsylvania – is to help determine the genetic sources of heart, lung, or blood conditions that also include the liver.
Understanding GWAS Studies
The GWAS studies map the genomes in hundreds of people as a way to look for genetic mutation patterns that differ from the genomes of healthy individuals. As GWAS studies map more genomes, they become more likely to find the correct genetic mutations that cause a disease. Once a panel of suspected mutations is built, stem cells from these individuals can be manipulated in culture dishes to differentiate into any of the body’s cells. The cells can be screened to learn more about the mutations and to test panels of drugs that might ultimately help treat patients harboring a disease.
Challenges in Differentiating iPSCs
Problems arise during the cell manipulation process. For example, iPSCs persistently refuse to mature uniformly into liver-like cells when fed growth factors. Traditionally, antibodies have been used to recognize features of maturity on the surfaces of cells and purify cells that are similar, an approach that has been crucial to stem cell research. However, available antibodies that recognize mature liver cells are scanty and tend to recognize many different kinds of cells. The many types of cells in mixed populations have diverse characteristics that can obscure underlying disease-causing genetic variations, which tend to be subtle.
Introducing Chemo Proteomic Cell Surface Capture (CSC) Technology
Instead of relying on antibodies, Duncan and his team embraced a new technology called chemo proteomic cell surface capture (CSC) technology. CSC technology allowed the researchers to map the most highly produced proteins on the surface of liver cells during the final stages of differentiation of stem cells into liver cells. The most abundant protein was targeted with an antibody labeled with a fluorescent marker and used to sort the mature liver cells from the rest.
Results of the New Purification Method
The procedure was highly successful: The team had a population of highly pure, homogeneous, and mature liver-like cells. Labeled cells had far more similar traits of mature hepatocytes than unlabeled cells. Pluripotent stem cells that had not differentiated were excluded from the group of labeled cells.
Implications for Liver Disease Treatment
“That’s important,” says Duncan. “If you’re wanting to transplant cells into somebody that has liver disease, you really don’t want to be transplanting pluripotent cells because pluripotent cells form tumors called teratocarcinomas.”
Duncan cautioned that transplantation of iPSC-derived liver cells is not yet ready for translation to the clinic, but the technology for sorting homogeneous liver cells can be used now to successfully and accurately model and study disease in the cell culture dish.
Future Directions
“We think that the ability to generate pure populations will get rid of the variability, and therefore really help us combine with GWAS studies to identify allelic variations that are causative of a disease, at least in the liver,” he says.
Publication Details
Researchers at the University of Minnesota (Minneapolis) and the Medical College of Wisconsin (Milwaukee) contributed to the study, published August 25, in Stem Cell Reports.=
- Published in Corporate News / Blog
Global Stem Cells Group Plans Bone Marrow Clinical Trials for Knee Osteoarthritis
Global Stem Cells Group has announced plans to hold clinical trials, pending IRB approval, for bone marrow stem cell treatments targeting knee osteoarthritis. The trials will be held in five GSCG facilities in the U.S. and South America, with 25 patients accepted for each location.
MIAMI, March 31, 2016—Pending Institutional Review Board (IRB) approval, Global Stem Cells Group, Inc. has announced plans to conduct a multi-center, placebo controlled clinical trial to measure the safety and effectiveness of the intra-articular application of freshly isolated bone marrow stem cells for the treatment of osteoarthritis.
The clinical trials, which will begin July 1, 2016 and run for one year, will be held in Global Stem Cell Group facilities in Buenos Aires, Argentina; Bogota, Colombia; Quito, Ecuador; Miami, Florida and Topeka, Kansas. Each center will accept 25 patients per clinical trial, and patients will receive a bone marrow stem cell injection in one knee and a placebo in the other knee..
 The trials are designed to investigate the possible beneficial effect of freshly harvested bone marrow stem cell applications on knee osteoarthritis patients in the control group. Patients will receive standard treatment of bone marrow stem cells intravenously, and will be monitored and assessed for any changes in clinical condition.
The trials are designed to investigate the possible beneficial effect of freshly harvested bone marrow stem cell applications on knee osteoarthritis patients in the control group. Patients will receive standard treatment of bone marrow stem cells intravenously, and will be monitored and assessed for any changes in clinical condition.
Knee osteoarthritis is a chronic, progressive condition affecting an increasing number of people, especially the elderly and obese. It is characterized by degeneration of the cartilage—the natural cushioning between joints inside the knee.
The condition is the result of the wearing away of cartilage. When this happens, the bones of the joints rub more closely against one another with less of the shock-absorbing benefits of cartilage, resulting in pain, swelling, stiffness and a decreased ability to move.
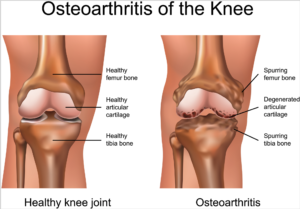 According to the Centers for Disease Control (CDC), knee osteoarthritis will affect 67 million people in the United States by 2030. While conventional treatments like physiotherapy or drugs offer temporary relief of clinical symptoms, total knee replacement is the closest treatment available for permanent relief, which requires invasive surgery, comes at a high cost and is not always successful. The latest advances in stem cell therapies for knee osteoarthritis are designed to restore cartilage function in the knee.
According to the Centers for Disease Control (CDC), knee osteoarthritis will affect 67 million people in the United States by 2030. While conventional treatments like physiotherapy or drugs offer temporary relief of clinical symptoms, total knee replacement is the closest treatment available for permanent relief, which requires invasive surgery, comes at a high cost and is not always successful. The latest advances in stem cell therapies for knee osteoarthritis are designed to restore cartilage function in the knee.
Global Stem Cells Group offers the most advanced protocols and techniques in cellular medicine from around the world.
Details of the protocol and eligibility criteria will be released upon IRB approval.
For more information on Global Stems Cell Group, visit the Global Stem Cells Group website, email bnovas(at)stemcellsgroup(dot)com, or call +1 305 560 5337.
About Global Stem Cells Group:
Global Stem Cells Group, Inc, is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
To view this press release live online, click here
###
- Published in Press Releases