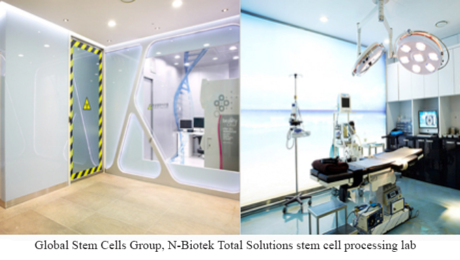
Global Stem Cells Group and N-Biotek Collaborate to Offer Turnkey Solutions for Highly Manipulated Stem Cell Culture Expansion Labs
MIAMI, Aug. 23, 2016 – Global Stem Cells Group and its subsidiary Adimarket announce a collaborative agreement with South Korean biomedical and lab equipment manufacturer N-Biotek to offer the Total Solution highly manipulated stem cell culture expansion lab, complete with equipment, training, facilities, stem cell processing center, clean rooms, project plan and more- a total turnkey lab solution for emerging stem cell businesses and industry professionals.
The announcement marks a new chapter in GSCG’s ongoing expansion, and commitment to bring stem cell therapies to

Physicians at work in Total Solution turnkey stem cell lab
Until now, Admarket has been GSCG’s online marketplace for quality regenerative medicine equipment and supplies for physicians and health care professionals, offering basic tools and products to facilitate in-office stem cell procedures. However, through N-Biotek, Adimarket now offers the Total Solution labs and all the accoutrements. From planning to building the facility, to training and consultation, Total Solution users are equipped in every way possible to walk in and begin using their fully-equipped biotechnology lab in short order.

Total Solutions clean room and stem cell lab equipment
The Stem Cell Total Solution is a comprehensive business solution that provides the entire stem cell processing system-from business planning to business procedures, a state-of-the-art cellular processing facility with bio safety clean rooms for stem cell processing, quality control rooms, core technology, cutting-edge equipment, installation, training-everything needed to launch a stem cell business or a enhance a medical research practice in a short period of time.
N-Biotek has been providing its Stem Cell Total Solution labs and consulting services, as well as stem cell processing technology management services, to universities, research centers, physicians and biotechnology enterprises in South Korea, China, Japan and Vietnam for years.
 Highly manipulated stem cells are critical to biotechnology researchers in order to advance both fundamental knowledge of the undifferentiated cells that have the remarkable potential to develop into many different cell types in the body during early life and growth, and to find ways to use them to treat medical and cosmetic conditions non-invasively. Additionally, in many tissues, stem cells serve as a sort of internal repair system, dividing essentially without limit to replenish damaged and dead cells for as long as the person or animal they inhabit is alive.
Highly manipulated stem cells are critical to biotechnology researchers in order to advance both fundamental knowledge of the undifferentiated cells that have the remarkable potential to develop into many different cell types in the body during early life and growth, and to find ways to use them to treat medical and cosmetic conditions non-invasively. Additionally, in many tissues, stem cells serve as a sort of internal repair system, dividing essentially without limit to replenish damaged and dead cells for as long as the person or animal they inhabit is alive.When a stem cell divides, each new cell has the potential to remain a stem cell or become another type of cell with a more specialized function, such as a muscle cell, a red blood cell, or a brain cell, and this allows researchers to manipulate these extraordinary reconstructionist micro-organisms to differentiate into other cell types that can be used to treat disease and injuries affecting parts of the body from which they did not originate. For instance, a stem cell harvested from body fat can be manipulated to take on the functions of a pancreatic cell, a heart cell or a retina cell. Researchers see unlimited potential in their ability to heal wounds, cure diseases, and even prevent birth defects.
 “Non-destructive manipulation of stem cells in a precise environment is key to facilitating discoveries within the biology and medical research communities,” says Benito Novas, CEO of Global Stem Cells Group. “Our collaboration with N-Biotek to deliver the Total Solution for highly manipulated stem cell culture expansion labs to emerging stem cell scientists is the next phase in GSCG’s mission to fulfill our promise of making stem cell-based therapies available to patients everywhere.
“Non-destructive manipulation of stem cells in a precise environment is key to facilitating discoveries within the biology and medical research communities,” says Benito Novas, CEO of Global Stem Cells Group. “Our collaboration with N-Biotek to deliver the Total Solution for highly manipulated stem cell culture expansion labs to emerging stem cell scientists is the next phase in GSCG’s mission to fulfill our promise of making stem cell-based therapies available to patients everywhere.“Stem cell manipulation resulting in cells that possess the characteristics necessary for successful differentiation, transplantation, and engraftment is critical to discovering new methods for treating illness and impairments,” Novas says.
Global Stem Cells Group and N-Biotek provide Total Solution users with everything they need to culture, expand and cryopreserve stem cells right away. Each system comes with manufacture patents and full certification. All products and
 equipment is made to comply with, or exceed standards required for CE Certification.
equipment is made to comply with, or exceed standards required for CE Certification.N-Biotek is the only company that builds the entire stem cell processing system for clients ready to begin a stem cell business, or establish a lab facility to enhance their medical or research practice.
To learn more about the N-Biotek Stem Cell Total Solution, visit the Global Stem Cells Group website at or the AdiMarket website, email bnovas@stemcellsgroup.com, or call 305-560-5337.
About Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
About Adimarket:
Adimarket, Inc., a subsidiary of Global Stem Cells Group, is a cost-competitive online marketplace for quality regenerative medicine equipment and supplies for physicians and health care professionals. Adimarket was founded to provide physicians and other health care professionals the tools they need to practice regenerative medicine in a medical office setting.
Motivated by a firm belief in the positive impact the practice of stem cell medicine can have when dispensed in a doctor’s office, Adimarket provides physicians with the tools they need to provide patients with cutting edge treatments. Adimarket’s experienced customer service representatives provide valuable guidance and advice regarding products relevant to individual practices.
About N-Biotek:
Since 1982, N-Biotek has been the leading manufacturer of biomedical and lab equipment worldwide. The Stem Cell Total Solution lab is a culmination of years of dedication to establish compact and customized products in developing the Handy Lab-a personal lab for medical, scientific and university professionals. N-Biotek is known for its high quality, uniquely designed biomedical products and equipment and competitive pricing. Over the years, the company’s products have earned an array of patents, and all meet international standards including CE, ETL, ISO and GMP. N-Biotek also offers real-time monitoring through IT technology.
Since 2010, N-Biotek has expanded significantly, starting new businesses including a stem cell processing system and biological clean room, GMP consulting, validation services and health care services for foreign consumers in order to maintain its lead in the life science field.
To view this press release live online, please click here
###
- Published in Press Releases
Global Stem Cells Group Announces Stem Cell Training in Madrid
[su_spacer]
Physicians at a recent stem cell training course
MIAMI, Aug. 16, 2016—Global Stem Cells Group and Stem Cell Training, Inc. will host a stem cell training course in Madrid, Dec. 6-7, 2016, in collaboration with Javier Garcia Alonso, MD of Clinica Castellana Norte in Madrid. Garcia Alonso is a specialist in plastic, reconstructive and aesthetic surgery.
The stem cell training course, available to qualified physicians, will focus on stem cell protocols in cosmetic, anti-aging, and aesthetic procedures.
The Adipose and Bone Marrow Stem Cell Training Course was developed for physicians and high-level practitioners to learn the process through a two-day, intensive, hands-on training program to arm participating physicians with clinical protocols and state-of-the-art techniques for isolating and re-integrating adipose- and bone marrow-derived stem cells. When used in aesthetic medicine therapies, patients walk away with more natural-appearing augmentation and enjoy a faster recovery period with little to no downtime.
Garcia Alonso will host and participate in training qualified physicians in stem cell harvesting, isolating and re-integration  techniques and protocols, during which stem cells are harvested from the patient’s own body and redistributed to areas of the body receiving augmentation.
techniques and protocols, during which stem cells are harvested from the patient’s own body and redistributed to areas of the body receiving augmentation.
Stem cell therapy is a promising treatment for facial rejuvenation and soft tissue augmentation because there are no incisional scars or complications associated with foreign materials. Demand for stem cell procedures s high worldwide—these effective procedures that do not involve going under the knife are driving the growth of stem cell protocols in the cosmetic industry, as more patients request new technologies that are less invasive than ever before.
Breast augmentati on, abdominoplasty, facelifts and liposuction continue to feature in the top most requested procedures for many clinics, surgeons say an increasing number of patients are seeking a more natural look with a quicker recovery time, according to Benito Novas, CEO of Global Stem Cells Group. In fact, non-surgical cosmetic procedures have grown exponentially over the past five years, and the trend is expected to continue to rise.
on, abdominoplasty, facelifts and liposuction continue to feature in the top most requested procedures for many clinics, surgeons say an increasing number of patients are seeking a more natural look with a quicker recovery time, according to Benito Novas, CEO of Global Stem Cells Group. In fact, non-surgical cosmetic procedures have grown exponentially over the past five years, and the trend is expected to continue to rise.
Surgeons are also seeing more diver sity when it comes to the type of procedures requested, including buttock enhancements, hair transplantation and anti-aging procedures on hands.
sity when it comes to the type of procedures requested, including buttock enhancements, hair transplantation and anti-aging procedures on hands.
“Patients are demanding procedures that are quick, easy, and provide a fast recovery with minimal downtime,” Novas says. “Stem cells provide the ability to rejuvenate and heal, making them a natural treatment for cosmetic and anti-aging applications.”
The stem cell training course will be offered through Global Stem Cells Group affiliate Stem Cell Training, Inc.
To learn more, visit the Global Stem Cells Group website, or the Stem Cell Training website, email bnovas(at)regenestem(dot)com, or call +1 305 560 5337.
About Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
About Stem Cell Training, Inc.:
Stem Cell Training, Inc. is a multi-disciplinary company offering coursework and training in 35 cities worldwide. The coursework offered focuses on minimally invasive techniques for harvesting stem cells from adipose tissue, bone marrow and platelet-rich plasma. By equipping physicians with these techniques, the goal is to enable them to return to their practices, better able to apply these techniques in patient treatments.
About Clinica Castellana Norte:
Clinica Castellana Norte in Madrid of
fers a comprehensive range of aesthetic and reconstructive procedures, from dietary advice to state-of-the-art stem cell therapies for cosmetic procedures. Clinica Castellana Norte operates under the highest quality standards, not only in medical and aesthetic treatments offered, but throughout the administrative process, ensuring privacy. Clinica Director Javier Garcia Alonso, M.D., is a member of the Spanish Society of Plastic Reconstructive and Aesthetic Surgery (SECPRE), and a member of the International Society of Plastic, Reconstructive and Aesthetic Surgery (IPRAS).
To view this press release live online, click here
###
- Published in Press Releases
Global Stem Cells Group to Honor Joseph Purita, MD at 3rd Annual International Symposium on Stem Cells and Regenerative Medicine in Buenos Aires
Global Stem Cells Group will honor Joseph Purita, MD at its 3rd Annual International Symposium on Stem Cells and Regenerative Medicine, to be held in Buenos Aires Sept. 28, 2016. Purita, an active contributor to the Miami-based biotech company, has been a key participant in GSCG’s growth since it launched in 2013.
[su_spacer size=”20″]

Joseph Purita, MD

Julio Ferreira, MD
The GSCG Symposium offers an opportunity for many of the world’s most respected authorities on stem cell and regenerative medicine to join Purita and other industry leaders to showcase advancements in research and therapies on a global level.

Duncan Ross, PhD
An interdisciplinary team of stem cell experts will provide a full day of high-level scientific lectures geared to medical professionals. Pioneers and luminaries in stem cell medicine will serve as featured speakers, led by keynote speakers Purita and Duncan Ross, PhD Ross, a GSCG Advisory Board faculty member, is a molecular biologist, immunologist and researcher, and the founder of Kimera Labs.
According to Benito Novas, Global Stem Cells Group CEO, the world-class event will showcase the historic advances in stem cell medicine achieved since the first symposium was held just two years ago in 2014.
“We’ve come so far since 2014, and this year’s symposium will highlight the strides Global Stem Cells Group has made in these two years,” Novas says. “And it is only fitting that we take the opportunity to acknowledge Dr. Purita’s contributions to our growth.
“This year, we will showcase how far stem cell therapies have come, and provide some of the most influential leaders like Dr. Purita, who understand the potential of these therapies and have dedicated their careers to making them a reality.”
Since 2014, Global Stem Cells Group has joined forces with some of the most prestigious regenerative medicine practitioners in South America as it focuses on growing its services throughout the global community. Stem cell therapies continue to revolutionize the anti-aging aesthetics industry while offering new hope for sufferers of serious chronic debilitating diseases.
To learn more about the 3rd Annual International Symposium on Stem Cells and Regenerative Medicine, visit the Global Stem Cells Group website, email bnovas(at)regenestem(dot)com, or call 305-560-5337.
About Global Stem Cells Group:
 Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
About Joseph Purita, MD
Joseph Purita, MD, a pioneer in the use of Stem Cell and PRP therapy for orthopedic conditions, graduated from Georgetown University Medical School and served his surgical internship at the University of Florida Medical Center. Following completion of a residency in orthopedic surgery at University of Miami-Jackson Memorial Hospital, where he served as chief administrative resident, Dr. Purita joined the Boca Raton Orthopaedic Group in 1981.
Purita is a Fellow, American Academy of Orthopedic Surgeons; Fellow, American College of Surgeons; member, American Medical Association; member, Southern Medical Association; member, Palm Beach Medical Society; member, Broward County Medical Society; member, Palm Beach Orthopedic Society, and member, Florida Medical Association. His certifications include the American Board of Orthopedic Surgery; American College of Orthopaedic Surgery; American Board of Pain Management, and the American Board of Regenerative Medicine.
Purita is an instructor and proctor of surgeons in the use of lasers in arthroscopic and orthopedic surgery at a variety of area hospitals, and heads GSCG’s Scientific Advisory Board.
To view this press release live online, click here
###
- Published in Press Releases
Julio Ferreira, M.D.
Julio Ferreira, M.D.
Global Stem Cells Group Advisory Board member
Julio Ferreira, M.D., is an internationally recognized and respected cosmetic surgeon and professor of medicine and aesthetic surgery at the Institute of Biomedical Sciences, University of Sao Paulo, Brazil;
As director of Clinica Ferreira in Argentina, Dr. Ferreira is dedicated to the combination of art and science in aesthetic medicine.
Dr. Ferreira serves as president of the South American Academy of Cosmetic Surgery and expert examiner at the International Board of Cosmetic Surgery. He also serves on the International Editorial Advisory Board of the American Journal of Cosmetic Surgery; former President of the International Academy of Cosmetic Surgery 2005/2007; a member and examiner, International Board of Cosmetic Surgery; Corresponding Fellow of the American Academy of Cosmetic Surgery; Honorary Member of the Spanish Society of Cosmetic Medicine and Surgery; Honorary Member of the Eurorusa Confederation of Societies of Aesthetic Plastic Surgery; Honorary Member of the Bulgarian Society of Cosmetic Surgery; Honorary Member of the Chilean Society of Cosmetic Surgery and Lipoplasty; Honorary Member of the Italian Society of Aesthetic Surgery, and Honorary Member of the French Society of Aesthetic Surgery.
- Published in Advisory Board Members
Joseph Purita, M.D.
Joseph Purita, M.D.
Global Stem Cells Group Advisory Board member
Joseph Purita, M.D., is a world-renowned orthopedic surgeon and stem cell pioneer, using cutting edge technology in regenerative medicine in conjunction with stem cell platelet rich plasma (PRP) therapy to treat orthopedic injuries and relieve pain. He is also a pioneer in the use of the laser in orthopedic surgery.
Named a U.S. News and World Report Top Doctor in 2012, Dr. Purita, a renown orthopedic and arthroscopic surgeon, heads Global Stem Cells Group’s Scientific Advisory Board. A pioneer in the use of stem cell and PRP therapy for orthopedic conditions, Dr. Purita has practiced with the Boca Raton Orthopaedic Group in Boca Raton, Florida since 1981. In 2012, Purita gained international attention when he treated New York Yankees pitcher Bartolo Colon’s ligament damage with stem cells, restoring the athlete’s injured shoulder and career. Purita has since treated an array of professional athletes with career-threatening injuries.
Dr. Purita is the director of the Institute of Regenerative and Molecular Orthopedics in Boca Raton, Florida, specializing in the use of stem cells and PRP injections for use in sports medicine and other musculoskeletal conditions. The Institute has treated some of the most prominent professional athletes from all major sports in both the U.S.. and abroad.
He is an instructor and proctor of surgeons in the use of lasers in arthroscopic and orthopedic surgery at a variety of area hospitals,
Dr. Purita is a Fellow, American Academy of Orthopedic Surgeons; Fellow, American College of Surgeons; member, American Medical Association; member, Southern Medical Association; member, Palm Beach Medical Society; member, Broward County Medical Society; member, Palm Beach Orthopedic Society, and member, Florida Medical Association. His certifications include the American Board of Orthopedic Surgery; American College of Orthopaedic Surgery; American Board of Pain Management, and the American Board of Regenerative Medicine.
He is Is Board Certified By The Following Organizations:
• American Board of Orthopedic Surgery
• American College of Orthopaedic Surgery
• American Board of Pain Management
Dr. Purita is a popular speaker at regenerative and orthopedic conferences worldwide.
- Published in Advisory Board Members
Global Stem Cells Group Announces Stem Cell Training Course Scheduled in Barcelona
Global Stem Cells Group and its subsidiary Stem Cell Training, Inc., have announced plans to hold a stem cell training course in Barcelona, Spain, Nov. 11 – 12, 2016. Orthopedic and cosmetic surgeon J. Victor Garcia Gimenez, M.D. will conduct the course for qualified physicians and medical professionals.
[su_spacer]

J. Victor Garcia Gimenez, M.D.
Garcia Gimenez, a member of the Global Stem Cells Group Advisory Board, first conducted the course for GSCG in 2014. The
course is part of the Miami-based biotech company’s growth in the European market.
The training course was developed for physicians and high-level practitioners to learn clinical protocols and state-of-the-art techniques for isolating and re-integrating adipose- and bone marrow-derived stem cells. Stem cells are harvested from the patient’s own body and redistributed to areas of the body receiving treatment. Patients experience an effective, non-invasive procedure, and a faster recovery period with little to no downtime.
Garcia Gimenez is a specialist in orthopedic and cosmetic surgery, president of Therapeutic Confrontations (CONFTERA), and practices cosmetic and anti-aging medicine, as well as aesthetic therapies in Barcelona. He is the president of the Spanish Society of Medicine and Cosmetic Surgery; co-director of the UAB-SEMCC; Chairman for Spain of the International Academy of Cosmetic Surgery, in addition to other medical and professional boards.
The stem cell training course will be offered through Global Stem Cells Group affiliate Stem Cell Training, Inc.
To learn more, visit the Global Stem Cells Group website, or the Stem Cell Training website, email bnovas(at)regenestem(dot)com, or call +1 305 560 5337.
About Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
About Stem Cell Training, Inc.:
 Stem Cell Training, Inc. is a multi-disciplinary company offering coursework and training in 35 cities worldwide. The coursework offered focuses on minimally invasive techniques for harvesting stem cells from adipose tissue, bone marrow and platelet-rich plasma. By equipping physicians with these techniques, the goal is to enable them to return to their practices, better able to apply these techniques in patient treatment.
Stem Cell Training, Inc. is a multi-disciplinary company offering coursework and training in 35 cities worldwide. The coursework offered focuses on minimally invasive techniques for harvesting stem cells from adipose tissue, bone marrow and platelet-rich plasma. By equipping physicians with these techniques, the goal is to enable them to return to their practices, better able to apply these techniques in patient treatment.
To view this press release live online, click here.
###
- Published in Press Releases
Global Stem Cells Group Announces Stem Cell Training Course in South Korea
(Pictured: bone marrow stem cells)
Global Stem Cells Group will host a stem cell training course in SVF and bone marrow aspiration techniques July 28-29, 2016.
MIAMI, July 28, 2016–Global Stem Cells Group, in collaboration with South Korean biomedical company N-Biotek will host a course in stromal vascular fraction (SVF) and bone marrow aspiration techniques for physicians at the N-Biotek headquarters in Gyeonggi-do Province of South Korea July 29 and 29, 2016.
The training course is part of a collaborative agreement between GSCG’s Adimarket division and N-Biotek, a worldwide  biomedical and lab equipment manufacturer, to promote and distribute their stem cell technology equipment throughout Latin America.
biomedical and lab equipment manufacturer, to promote and distribute their stem cell technology equipment throughout Latin America.
The two-day, hands-on training covers the latest technology and procedures in SVF and bone marrow stem cell techniques. Practitioners learn skills that can be used to treat patients in their practices, and for career advancement. The SVF and bone marrow aspiration course was developed for physicians and high-level practitioners to learn techniques in harvesting and reintegrating stem cells derived from adipose tissue and bone marrow. The objective of the training teach effective, in-office regenerative medicine techniques.
 N-Biotek develops a range of custom lab products including the Esfomi cosmetic line, and the Stem Cell Total Solution for emerging stem cell businesses.
N-Biotek develops a range of custom lab products including the Esfomi cosmetic line, and the Stem Cell Total Solution for emerging stem cell businesses.
N-Biotek is the only company that builds the whole stem cell processing system for partners ready to begin work in the stem cell industry. N-Biotek meets every need for stem cell clinicians, including biological clean room construction, equipment installation and stem cell processing consulting.
N-Biotek currently distributes medical equipment and services to facilities and professionals in more than 100 countries.
For more information, visit the Global Stem Cells Group website, email bnovas@stemcellsgroup(dot)com, or call +1 305 560 5337.
About Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
About Adimarket:
Adimarket, Inc., a division of the Global Stem Cells Group, is a cost-competitive online marketplace for quality regenerative medicine equipment and supplies for physicians and health care professionals.
Adimarket was founded to provide physicians and other health care professionals the tools they need to practice regenerative medicine in a medical office setting. Motivated by a firm belief in the impact the practice of stem cell medicine can have when dispensed in a doctor’s office, Adimarket provides physicians with the tools they need to provide patients with cutting edge treatments.
Adimarket’s experienced customer service representatives provide valuable guidance and advice regarding products relevant to individual practices.
About N-Biotek:
N-Biotek, Inc., founded in 2000 and located in the Gyeonggi-do Province of South Korea, is a leading manufacturer and supplier of bio-technology-related laboratory equipment. N-Biotek delivers high quality biomedical equipment to more than 100 countries.
To view this press release live online, click here
###
- Published in Press Releases
New Guidelines for Stem Cell Research and Therapies Aim to Protect Patients from Charlatan Quackery
Introduction
Stem cell research has advanced significantly, leading to a myriad of treatment options. However, the field faces challenges from unscrupulous providers and premature publicity.
Professional Guidelines for Responsible Stem Cell Research
International Society for Stem Cell Research (ISSCR)

The ISSCR leads in setting high standards for translational stem cell research:
- Emphasizes rigorous preclinical evidence and peer review.
- Highlights the importance of IRB review and comprehensive informed consent.
International Society for Cellular Therapy (ISCT)
The ISCT expands its scope beyond stem cells, advocating for broader cell-based interventions:
- Focuses on defining scientific evidence and regulatory practices.
- Addresses implications across clinical practice and commercialization.
Development of New Guidelines
Terminology and Scientific Evidence
Efforts are underway to standardize terminology and define scientific evidence levels critical for ethical and effective stem cell therapy.
ISSCR’s 2016 Guidelines Update
In 2016, ISSCR updated guidelines cover:
- Emerging technologies like gene editing and induced pluripotent stem cells (iPSCs).
- Upholding ethical standards such as the “14-day rule” for embryo experimentation.
Key Topics Addressed in the Revised Guidelines
The updated guidelines include:
- Oversight processes for embryo research and mitochondrial replacement therapy.
- Standards for preclinical and clinical research, emphasizing safety and efficacy.

Advancements in Stem Cell Research
Stem cell therapies show promise in treating a range of conditions, leveraging pluripotent stem cells for tissue repair and genetic disease treatments.
Conclusion
Stem cell research continues to evolve responsibly, offering hope for future medical advancements while safeguarding patient interests against fraudulent practices.
- Published in Corporate News / Blog
Global Stem Cells Group Launches Two Stem Cell Treatment Centers in Arica and Iquique, Chile

Global Stem Cells Group announces the launch of two new stem cell treatment clinics in the cities of Arica and Iquique in northern Chile. The facilities are part of the international biotech company’s expanding presence in Latin America.
MIAMI, May 31, 2016—Global Stem Cells Group, a leading international biotechnology company, announces the launch of operations at two new GSCG clinics in the cities of Arica and Iquique in northern Chile. The facilities are part of GSCG’s expanding presence in Latin America.
Both the Arica and Iquique clinics offer the most advanced protocols and techniques in stem cell medicine to patients from around the world.

The clinics are headed by stem cell specialists Victor Perez, M.D., and Duval Aguirre, M.D., and will offer treatments in chronic degenerative conditions, Type 2 diabetes, COPD, traumatology and sports medicine.
Global Stem Cells Group, has been expanding its clinical presence throughout Latin America and worldwide by partnering with qualified physicians experienced in stem cell therapies to open new clinics. The new Arica and Iquique clinics are certified for the medical tourism market.
Global Stem Cells Group is committed to the highest standards in service and technology, expert and compassionate care, and a philosophy of exceeding the expectations of their international patients.
For more information, visit the Global Stem Cells Group website, Email bnovas@stemcellsgroup.com, or call 305-560-5337.
About the Global Stem Cells Group:

Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions.
With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Groups corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCGs six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
To view this press release live online, click here
###
- Published in Press Releases
Global Stem Cells Group Subsidiary Adimarket to Launch Progenikine™ SVF Closed System

Global Stem Cells Group subsidiary Adimarket is preparing to launch Progenikine™, a new SVF closed system kit utilizing EmCyte technology, containing all the elements necessary to process adipose tissue and obtain stromal vascular fraction in a sterile environment.

MIAMI, May 31—Adimarket, a subsidiary of Global Stem Cells Group, Inc. is preparing to launch Progenikine™, its new and approved SVF closed system kit using EmCyte technology, and is expected to be available to physicians in July, 2016. The Progenikine kit contains all the elements necessary to process adipose tissue and obtain stromal vascular fraction (SVF) in a closed environment.
Adipose derived stem cells (ASCs) are used by physicians for a variety of indications. Most commonly, ASCs are isolated at the point of care from lipoaspirate (derived from liposuction) tissue as the stromal vascular fraction (SVF), harvested from the patient and immediately administered to the patient as an injection, or used to enrich fat grafts. Isolation of ASCs from adipose tissue is a relatively simple process performed routinely in cell biology laboratories, but isolation at the point of care for immediate clinical administration requires special methodology to prevent contamination, ensure integrity of the clinical procedure, and comply with  regulatory requirements.
regulatory requirements.
Developed in conjunction with Patrick Pennie, Emcyte CEO, and Maritza Novas Director of Research and Development for Global Stem Cells Group, Progenikine fuses elements from Emcyte systems with the Global Stem cells Group SVF protocols. The kit can provide a low cost, rapid and
simple alternative to traditional methods of isolating ASCs, particularly when smaller quantities are needed.
“We’re excited to launch the Progenikine kit, the newest product designed to help Global Stem Cells Group’s fulfill its m
ission to provide accessible products to our member clients,” says Benito Novas, CEO of Global Stem Cells Group. “These are the milestones that bring us closer to ensuring that more patients will be able to gain access to stem cell therapies.”
To learn more about the Progenikine kit, visit the Global Stem Cells Group website, email bnovas@stemcellsgroup.com, or call 305-560-5337.
About Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions.
With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Groups corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCGs six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About Adimarket:
Adimarket, Inc., a division of the Global Stem Cells Group, is a cost-competitive online marketplace for quality regenerative medicine equipment and supplies for physicians and health care professionals.
Adimarket was founded to provide physicians and other health care professionals the tools they need to practice regenerative medicine in a medical office setting. Motivated by a firm belief in the impact the practice of stem cell medicine can have when dispensed in a doctor’s office, Adimarket provides physicians with the tools they need to provide patients with cutting edge treatments.
Adimarket’s experienced customer service representatives provide valuable guidance and advice regarding products relevant to individual practices.
About Emcyte:

Fort Myers, Florida-based EmCyte Corporation is a leader in autologous cellular biologics with the GenesisCS Component Concentrating Systems. These systems provide patients with the best opportunity for rapid recovery an
d provide practitioners with the most advanced clinical point of care experience. EmCyte systems are developed to meet every clinical requirement, giving the physician better clinical choices. EmCyte devices have been independently reviewed and show to produce buffycoat concentrations of 6x to greater than 10x baseline in 7mLs, with yields ranging from 70 percent to greater than 90 percent
EmCyte technology allows for the safe extraction of concentrated platelets and other regenerative cell types from the patient’s own blood. These cells are then re-suspended in a small volume of the patient’s blood plasma and then applied to the treatment site.
To view this release live online, click here
###
- Published in Press Releases