Global Stem Cells Group Partners with StemBio to Advance Regenerative Medicine Research and Development
Miami, Florida— Global Stem Cells Group, a leader in regenerative medicine, is thrilled to announce a strategic partnership with StemBio, a renowned leader in the regenerative medicine landscape in Turkey. This partnership aims to propel research and development, conduct clinical trials, and create new products and protocols for the advancement of regenerative medicine.
StemBio, a trailblazer in Turkey, possesses all the necessary licenses and state-of-the-art infrastructure for regenerative medicine, including Bone Marrow Derived MSC, Adipose Derived MSC, Umbilical cord, placenta, Wharton jelly Derived MSC, Synovial Derived MSC, and more. We believe this diverse range of resources demonstrates StemBio’s commitment to excellence, making them one of the foremost facilities for regenerative medicine in Turkey.
This collaboration brings together two powerhouses in the regenerative medicine arena, aiming to break new ground in the research, development, and application of regenerative therapies. We believe the collective expertise and resources of StemBio and Global Stem Cells Group are set to drive innovation and advancements in regenerative care, benefiting patients worldwide.
“We are excited to partner with StemBio and leverage their expertise in regenerative medicine. This partnership should accelerate our mission to provide cutting-edge regenerative solutions to a global audience,” said Benito Novas, head of relationships at Global Stem Cells Group.
We believe the partnership between StemBio and Global Stem Cells Group signifies a significant milestone in the regenerative medicine field. As they combine their strengths and knowledge, they are committed to pushing the boundaries of what is possible in regenerative care and making a lasting impact on patients’ lives.
About Global Stem Cells Group:
The Global Stem Cell Group is a family of several companies focused on stem cell medicine and research. The company uses its network to bring leadership in regenerative medicine training, research, and patient applications.
GSCG’s mission is to allow physicians to present the benefits of stem cell medicine to patients worldwide. The company also partners with policymakers, educators, and regulators to promote regenerative medicine.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV. https://finance.yahoo.com/quote/mssv/
To learn more about Global Stem Cells Group, Inc.’s companies visit our website www.stemcellsgroup.com or call +1 305 560 5331
About StemBio:
StemBio is a renowned regenerative medicine facility in Turkey, equipped with cutting-edge infrastructure and a commitment to excellence. Holding the necessary licenses for cord blood and tissue banking, as well as advanced engineering solutions, StemBio is a leader in the regenerative medicine landscape.
Safe Harbor Statement: Statements in this news release may be “forward-looking statements”. Forward-looking statements include, but are not limited to, statements that express our intentions, beliefs, expectations, strategies, predictions, or any other information relating to our future activities or other future events or conditions. These statements are based on current expectations, estimates, and projections about our business based partly on assumptions made by management. These statements are not guarantees of future performance and involve risks, uncertainties, and assumptions that are difficult to predict. Therefore, actual outcomes and results may and are likely to differ materially from what is expressed or forecasted in forward-looking statements due to numerous factors. Any forward-looking statements speak only as of the date of this news release, and The Global Stem Cells Group undertakes no obligation to update any forward-looking statement to reflect events or circumstances after the date of this news release. This press release does not constitute a public offer of any securities for sale. Any securities offered privately will not be or have not been registered under the Act and may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements.
- Published in Press Releases
Atherosclerosis: The Most Common Form of Arterial Occlusive Disease in Adults
Introduction to Atherosclerosis
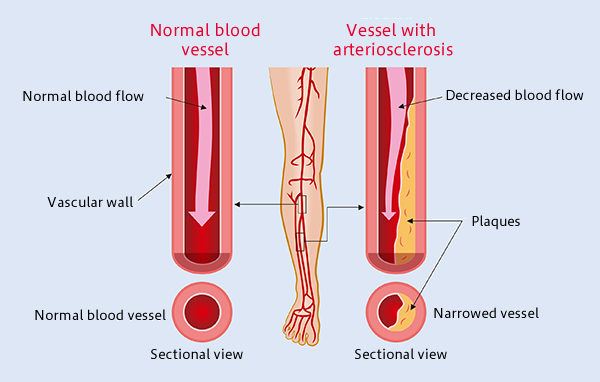
Atherosclerosis is the most common form of arterial occlusive disease in adults. About 15 percent of adults over 55 years of age suffer from critical ischemia, the most severe form of this disease.
Due to the gradual aging of the population and the growing number of people in their third age group, a number of studies have been conducted in order to improve the prognosis of atherosclerosis obliterans and to find alternatives to the mutilation of the extremities. As a general rule, chronic ischemia of the lower limbs should be treated to alleviate symptoms, particularly pain, prevent disease progression, and reduce the rate of amputations. In most patients with critical ischemia, the main goal is to preserve the affected limb.
Atherosclerosis Obliterans Grade IV
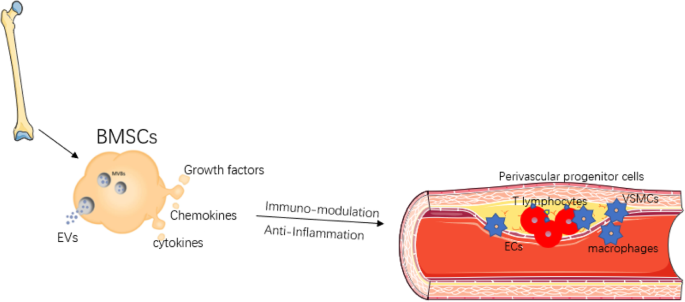
The development of regenerative medicine is closely linked to the development of new knowledge about embryonic and adult stem cells, as well as the regenerative and therapeutic potential of stem cell therapy. The use of adult stem cells in the treatment of peripheral artery diseases has been demonstrated as a therapeutic agent for inducing angiogenesis. Recent preclinical studies, as well as pioneering clinical studies, indicate that bone marrow-derived mononuclear cells (MBMCs) can enhance tissue vascularization in ischemic limbs, with results similar to those obtained with peripheral blood stem cells supply.
Case Studies and Research
Cuba presented the first studies carried out in 2004 at the Institute of Hematology of the “Enrique Cabrera” hospital in Havana City, which achieved encouraging clinical results and had very few adverse effects in recent years.
A progressive rise in the accumulated experience with stem cells was also observed in Pinar del Rio in 2005, as the first 10 cases were carried out. The rising ease of obtaining this type of cell has made research and applications with these cells advance rapidly with great expectations in terms of clinical application.
Research Findings on Atherosclerosis Obliterans Grade IV
A study published by Dia-Diaz, et al. in the Journal of Medical Sciences of Pinar del Rio examined 296 patients with grade IV atherosclerosis obliterans between 2009 and 2019. During the study, autologous stem cells were injected intramuscularly from peripheral blood. Within four weeks, pain relief was observed, as well as an increase in the pain-free claudication distance. Angiography after treatment revealed collateral vessel formation. The limb was saved in 201 patients (68%), while 95 cases (32%) presented amputation criteria. Complications were not reported following the procedure.
The study demonstrated the effectiveness of the implantation of autologous stem cells obtained from peripheral blood, as well as the favorable evolution of patients, clinical improvement of rest pain, walking distance without claudication, and ankle-brachial pressure index.
We still need to explore a lot of ground in terms of these and other conditions. You can learn more about regenerative medicine and stem cells by enrolling in our international certification program at www.issca.us.
- Published in Corporate News / Blog
ISSCA Members to Present in the XXV Congreso Internacional de Medicina, Cirugia Estética y Obesidad
The XXV Congreso Internacional de Medicina, Cirugia Estética y Obesidad will take place in CDMX, México on July 13,14 and 15, 2022. The International Society for Stem Cell Application (ISSCA) will be actively taking part in the congress. The ISSCA is a multidisciplinary community of physicians and scientists with a mission to advance the science, technology, and practice of regenerative medicine to treat diseases and lessen human suffering through science, technology, and regenerative medicine. Several ISSCA members will be presenting or giving demonstrations on the latest protocols and technologies in regenerative medicine.
- Benito Novas, the managing director of the ISSCA from the USA, will give a talk about Digital Marketing in Cash-Based Practices. Benito Novas is a global entrepreneur, manager, and keynote speaker, who specializes in marketing focused on biotechnology, life sciences, and healthcare development. He has served as the director of the ISSCA since 2016. His published books in Aesthetic Practice and Digital Marketing can be found here. In his presentation this July, he will share his visionary approach to healthcare management and regenerative medicine. Congress attendees will have the opportunity to learn why doctors must use digital marketing strategies to grow a successful practice. Benito Novas will provide tips to attendees on how to capitalize on social media trends to grow practice influence.
- Dr. Maritza Novas, the ISSCA director of education from the USA, will talk about Allogeneic Therapies and Stromal Cell Exosomes. The use of allogeneic therapy is one of the most attractive alternatives to autologous products and is of utmost interest to researchers in recent years. Exosomes serve as mediators for cell-to-cell communication and can be used as cell-free therapeutics for their special characteristics. Dr. Maritza Novas has been in aesthetic and anti-aging medicine since 2001. She is globally recognized for her work in regenerative medicine and dedicated service in education.
- Dr. Silvina Pastrana, the Argentina chapter director of the ISSCA from Argentina, will present on Fundamentals of Cellular Therapies and Mesenchymal Stem Cells (MSC). Dr. Pastrana heads a staff of medical specialists in orthopedics, rheumatology, medical clinic, and cosmetic surgery, performing procedures that incorporate stem cell therapies. She has been serving as a staff surgeon for the Hospital Dr. Prof. Luis Güemes for 21 years.
Besides the above presentations, look forward to sessions in the practical portion of the congress, where the following ISSCA members will show the latest protocols and technologies in regenerative medicine:
- Dr. Julio Ferreira (Argentina), Cosmetic Surgery / Aesthetic Medicine
- Dr. Andrea Lapeire (Argentina), Aesthetic Medicine
- Dr. Maritza Novas (USA), ISSCA Director of Education in the USA
- Dr. Silvina Pastrana (Argentina), Chapter Director of ISSCA in Argentina
The ISSCA members are the leaders in setting standards and promoting excellence in the field of regenerative medicine, related education, certification, research, and publications. The Global Stem Cells Groups (GSCG) is a group of companies, including the ISSCA and other members, dedicated to facilitating stem cell research and medicine. Making the benefits of stem cell medicine a reality for both doctors and patients worldwide is the goal of the GSCG. To learn more, visit our sites at the ISSCA and the GSCG.
- Published in Press Releases
How much does exosome therapy cost?
Exosome therapy is the new buzz in the regenerative medicine industry because of how it can repair and regenerate your cells and tissues.
Exosome therapy is safer compared to other cellular therapy because it’s a cell-free therapy with no risk of rejection.
Exosome therapy will be beneficial to you if you’re dealing with conditions such as sports injuries, tissue regeneration, hair loss, erectile dysfunction, chronic pain, and many other applications.
In this article, you’ll learn about the cost of exosome therapy and how you can benefit from it.
How the Cost of Exosome Therapy is Determined
All cells produce exosomes, which are microvesicles that contain biochemical and genetic information.
The cost of an exosome product (used in exosome therapy) will depend on the type of cell line (raw tissue source) used to extract the exosomes.
The first factor to determine the cost of an exosome product is the quality of the tissue source.
The most commonly used tissue types are cord blood, amniotic fluid, and mesenchymal cell cultures.
Exosomes derived from mesenchymal cell cultures are the most difficult to obtain but offer the greatest therapeutic potential.
How Much Does Exosome Therapy Cost?
The average cost of exosome therapy is $4,900, but the price can range from $3,500 to $6,500.
It’s also important to note that the price depends on your specific needs and your treatment plan, as decided by the doctor.
The doctor will schedule a consultation with you to determine your personalized treatment plan.
The exosome therapy can either be given as an IV infusion or as localized injections, depending on the purpose of the therapy.
Exosomes are very useful to revitalize, rejuvenate, restore, and reduce inflammations in the body.
Benefits of Exosome Therapy
Hair Loss Therapy
If you’re in the early stages of hair loss, exosome therapy can help regenerate your hair whether you’re a man or woman. After exosome therapy, you’ll start seeing new hair growth in as little as two to three months, with significant results showing six months to one year later.
Chronic Pain
If you’re experiencing chronic pain due to degenerative conditions such as arthritis, exosomes can help subdue the pain by regenerating the cells and helping the body work better.
Degenerative Conditions
For degenerative medical conditions such as osteoarthritis and musculoskeletal injuries, exosome therapy can help your body repair the damage done to your cells, prevent further deterioration, and help you feel better.
Skin Therapy
Exosome therapy can reduce inflammation in the skin by improving its strength and elasticity.
Anti-Aging
If you would like to retain your youthful glow, exosome therapy can make you feel young again by rejuvenating your skin and reversing the aging process in your cells.
Where Can You Get Exosome Therapy?
Cellular Hope Institutes provide exosome therapy for patients looking for better outcomes for various conditions.
The exosomes used at Cellular Hope Institute are obtained from umbilical cord tissue discarded after birth, which means these exosomes have not been exposed to any contaminants or toxic agents, ensuring a higher capacity to regenerate your cells and tissues.
- Published in Corporate News / Blog
Culture Expanded MSCs
Mesenchymal Stem Cells (MSCs) are the most commonly used cells in stem cell therapy and regenerative medicine due to their high multi-potency. MSCs can be isolated from different tissues in the body.
In this article, you’ll learn about culture-expanded MSCs, how MSCs can be expanded, the potency of MSCs, and the types of cells they can differentiate into.
What Are Culture Expanded Mesenchymal Stem Cells?
Mesenchymal stem cells are highly potent cells used for cellular therapy and are isolated from different parts of the body. MSCs can be used to improve patient outcomes in diseases and conditions such as autoimmune diseases, degenerative diseases, nerve damage, diabetes mellitus, bone problems, etc.
For every patient, millions of MSCs are needed, and the exact amount varies according to disease, route of administration, administration frequency, weight, and age of the patient.
MSCs are expanded in a culture medium on a large scale to obtain the required quantity of cells needed for cellular therapy.
Culture Expanded MSCs: How Does It Work?
Expanding MSCs in a medium involves a step-by-step process of isolation and expansion.
Mesenchymal Stem Cells Isolation
MSCs can be isolated from different tissues in the human body such as adipose tissues, dental pulp, human bone marrow, umbilical cord tissue, umbilical cord blood, peripheral blood, and synovium.
MSCs are expanded in culture to increase their yield and amplify their desired functions and potency.
Although the population of MSCs obtained will vary from donor to donor, here are some steps to follow:
- Acquire fresh tissue extracts in strictly aseptic conditions to maintain purity.
- To remove any cell clusters, filter the cell suspension with a 70-mm filter mesh.
- Use a centrifuge to roll the cells for about 5 minutes at 500g.
- Suspend the cells again to measure the cell viability and yield using Trypan blue exclusion.
- Use T75 culture dishes to culture the cells in 10 mL of complete MSC medium at a density of 25 × 10^6 cells/mL. Incubate the plates at 37°C with 5% CO2 in a humidified chamber without any interruption.
- After 3 hours, remove the non-adherent cells by changing the medium and replacing it with 10 mL of fresh complete medium.
- After an additional 8 hours of culture, add 10 mL of fresh complete medium as a replacement for the existing medium. Repeat this step every 8 hours for up to 72 hours of initial culture.
- Cells can be frozen in MSC growth media plus 10% DMSO (D2650) at a density of 2X10^6 cells/vial.
Expansion of Mesenchymal Stem Cells in a Culture Medium
Culture-expanded MSCs undergo various stages from the preparation of the culture plate, thawing of MSCs, and the actual expansion of MSCs.
The reason behind the cultural expansion of MSCs is to get them to differentiate into other cell types such as osteoblasts, adipocytes, and mesenchymal stromal cells.
Preparation
To expand MSCs in a culture medium, you need culture ware. You can get one plastic or glassware plate and coat it with a sufficient amount of 0.1% gelatin. Don’t forget to aspirate the gelatin solution from the coated plate or flask before you use it.
Thawing of Mesenchymal Stem Cells
- After the recommended culture medium and coated culture ware is ready and on standby, remove the vial of MSCs from liquid nitrogen and incubate in a 37°C water bath until all the cells are completely thawed. The extent of completely thawed frozen cells and how fast they thaw determines cell viability.
- Once the cells have thawed completely, disinfect the walls with 70% ethanol before proceeding to the next step.
- Place the cells in a hood, and carefully transfer the cells to a sterile tube with a pipette (1 or 2 mL pipette), doing this to prevent bubbles.
- Add drops of MSC expansion medium that have been pre-warmed to 37°C to the tube containing the MSCs.
- Mix the suspension slowly by pipetting up and down two times while avoiding any bubbles.
- Place the tube in a centrifuge and centrifuge the tube at 300 x g for 2-3 minutes to roll the cells, avoiding vortexing the cells.
- Decant as much of the supernatant as possible to remove residual cryopreservative (DMSO).
- Suspend the cells in a total volume of 10 mL of MSC Expansion Medium again or any alternative of choice, pre-warmed to 37°C, containing freshly added 8 ng/mL FGF-2 (F0291).
- Place the cell suspension onto a 10-cm tissue culture plate or a T75 tissue culture flask.
- Maintain the cells in a humidified incubator at 37°C with 5% CO2.
- The next day, exchange the medium with fresh MSC Expansion Medium (pre-warmed to 37°C) containing 8 ng/mL FGF-2. Replace with fresh medium containing FGF-2 every two to three days thereafter.
- Isolate the cells when they are approximately 80% confluent, using Trypsin-EDTA, and passaged further or frozen for later use.
Functions of Culture Expanded MSCs
MSCs are required to be expanded in order for them to be used clinically for therapeutic purposes.
Culture-expanded MSCs can be induced to differentiate into adipocytes, osteocytes, hepatocytes, chondrocytes, tenocytes, and cardiomyocytes.
Due to their potential to differentiate into different kinds of cells in the body, MSCs can be used to manage liver problems, heart problems, joint and bone problems, etc.
MSCs are also used in tissue regeneration and modulation of the immune system. They possess anti-apoptotic, angiogenic, anti-fibrotic, and anti-oxidative properties.
However, the quantity of MSCs isolated from body tissues is not enough for clinical and therapeutic applications.
This is why MSCs are expanded in culture to increase their yield for desired therapeutic effect.

- Published in Corporate News / Blog
Meso Numismatics Closes Global Stem Cell Group Acquisition
LAS VEGAS, NV, August 18, 2021 (GLOBE NEWSWIRE) — via NEWMEDIAWIRE — Meso Numismatics, Inc. (“Meso Numismatics” or the “Company”) (MSSV), a technology and numismatic company specializing in the Meso Region, including Central America and the Caribbean, MSSV is pleased to announce that it has closed its acquisition of Global Stem Cells Group. “We are pleased to have finally been able to close on this acquisition,” said David Christensen, President and CEO of MSSV,” We are now well positioned to enter into the growing global Regenerative Medicine industry.”
Global Stem Cells Group (Global) is a premier Regenerative Medicine company that specializes in cutting edge stem cell research, current clinical applications, and physician training. Global licenses its intellectual property and name to medical professionals around the world providing them with necessary equipment and cellular therapy products needed to perform safe and effective Regenerative medicine related treatments. Global has one of the larges physician membership networks in the world with 29 offices in more than 25 countries. The worldwide Regenerative Medicine industry is a multi billion-dollar industry projected to have double digit annual growth rates over the next decade. For more information on Global Stem Cells Group please follow the link www.stemcellsgroup.com
Please read this press release in conjunction with the 8K that was filed today on www.sec.gov.
MSSV has also renegotiated its June 22, 2021 $10.5M financing, of which $8.2M is earmarked for the Lans Holdings Escrow as per the 8k filed June 24th, 2021, as follows: the warrant maturity has been changed to 7 years instead of 3 years and the warrant shall be locked up for the first 2 years whereas during the lock up period the warrant will not be able to be exercised.
This press release should be read in conjunction with all other filings on www.sec.gov
For more information on Global Stem Cells Group please visit: www.stemcellsgroup.com
About Meso Numismatics: Meso Numismatics, Corp is an emerging numismatic and technology company specialized in the Meso Region, including Central America and the Caribbean. The Company has quickly become the central hub for rare, exquisite, and valuable inventory for not only the Meso region, but for exceptional items from around the world. With the Company’s breadth of business experience and technology team, the Company will continue to help companies grow. Meso has now added bio-technology to it’s portfolio and will continue to grow the company in this new direction.
This press release should be read in conjunction with all other filings on www.sec.gov
For more information on Global Stem Cells Group please visit: www.stemcellsgroup.com
About Meso Numismatics: Meso Numismatics, Corp is an emerging numismatic and technology company specialized in the Meso Region, including Central America and the Caribbean. The Company has quickly become the central hub for rare, exquisite, and valuable inventory for not only the Meso region, but for exceptional items from around the world. With the Company’s breadth of business experience and technology team, the Company will continue to help companies grow.
Forward Looking Statements
Some information in this document constitutes forward-looking statements or statements which may be deemed or construed to be forward-looking statements, such as the closing of the share exchange agreement. The words “plan”, “forecast”, “anticipates”, “estimate”, “project”, “intend”, “expect”, “should”, “believe”, and similar expressions are intended to identify forward-looking statements. These forward-looking statements involve, and are subject to known and unknown risks, uncertainties and other factors which could cause the Company’s actual results, performance (financial or operating) or achievements to differ from the future results, performance (financial or operating) or achievements expressed or implied by such forward-looking statements. The risks, uncertainties and other factors are more fully discussed in the Company’s filings with the U.S. Securities and Exchange Commission. All forward-looking statements attributable to Meso Numismatics, Inc., herein are expressly qualified in their entirety by the above-mentioned cautionary statement. Meso Numismatics, Inc. disclaims any obligation to update forward-looking statements contained in this estimate, except as may be required by law.
For further information, please contact:
Investor.relations@mssvinc.com
Telephone: (800) 956-3935
- Published in Press Releases
GCell: The Newest All-In-One Solution in Regenerative Medicine
Are you a physician currently utilizing adipose tissue but growing tired of the time-consuming, arduous procedure? Are you having problems finding reagents that allow you to isolate stem cells? If so, GCell might be the solution for you.
What is GCell?
GCell is a revolutionary new machine poised to become the future of adipose stem cell processing. Its compactness and short processing time make it a valuable implement in any regenerative medicine practitioner’s office. GCell employs a process of mechanical breakdown, which shortens the treatment duration and keeps it within legal minimal-manipulation regulations.
How Does GCell Work?
Through a system of extremely small blades and filters, GCell homogenizes a sample of fat taken from anywhere in the body, turning it into a slurry of growth factors, proteins, and other components. GCell then filters out the stromal vascular fraction cells and uses a process called photoactivation to increase their vitality. The end result is a final product that can be administered to patients within forty minutes from the sample’s harvesting. This is a significant improvement over the previous, multi-hour long treatments that physicians have grown accustomed to, and this shortened timespan is greatly appreciated by both doctors and patients.

Why GCell?
GCell offers a single-session treatment, meaning patients only need to visit their clinic once without requiring follow-up treatments. This machine combines the convenience of its small size and lightweight build with the ability to accelerate the stem cell harvesting process. Additionally, GCell is designed to be an entirely enclosed system, ensuring samples remain in a sterile environment, which preserves the viability of samples and ensures positive outcomes for patients.
GCell: The Newest All-In-One Solution in Regenerative Medicine
GCell combines a grinder, a filter, and a photoactivation device, offering a turnkey solution that can take a physician from harvesting a fat sample to administering an injectable regenerative medicine therapy in less than an hour. GCell is especially useful for clinics just beginning to implement regenerative medicine into their practice, with its all-inclusive approach to stem cell isolation setting it a cut above the rest.

GCell’s Single Session Treatment Protocol
Unlike many other cellular therapies on the market, GCell provides a single-session treatment, eliminating the need for patients to return after their original treatment day. Its portability, small stature, and ability to accelerate the stem cell harvesting process make it ideal for clinics new to regenerative medicine, taking much of the rigor out of adipose-derived stem cell harvesting.
- Published in Corporate News / Blog
Global Stem Cells Group Announces Partnership with Bioscience Cell Factory
Just earlier this week, Global Stem Cells Group has signed a historic agreement with a company known as Bioscience Cell Factory– this Dubai-based healthcare company will allow GSCG to act as their representatives operating in both the Middle East and Latin America. Through the acquisition of proprietary branding rights Global Stem Cells Groups will become committed to promote the widespread use of cellular therapy treatments that utilize Mesenchymal Stem Cells (MSCs).
This partnership is groundbreaking in nature, and promises to lead to a wide proliferation of cellular therapies in both the Middle East and Latin America, two regions that are rapidly developing, with a constantly-advancing standard of medical care. Global Stem Cells Group will be clinically equipped with the highest standards and quality procedures as set forth by the Bioscience Cell Factory, resulting in nothing but the best treatment methods available for patients around the world.

“I’m extremely excited about this partnership,” Said Benito Novas, CEO of the Global Stem Cells Group, “Bioscience Cell Factory is one of the most professional and scientifically-focused teams that I’ve ever had the pleasure of working with– I am looking forward to the start of what will be an extremely beneficial professional relationship that will provide our global reach with the quality of Bioscience’s laboratory,”
Indeed, the two firms are also announcing plans to do research together into the nature of adult Mesenchymal Stem Cells, and the benefits that they hold for the medical field. Through the training of physicians and the handling of cell samples in their laboratory, there are high hopes for the advancement of the field of regenerative medicine research. As one of the representatives of the Bioscience Cell Factory abroad, Global Stem Cells Group will further its goal of being a global leader in the regenerative medicine field.
About Bioscience Cell Factory
Bioscience Clinic is a global healthcare company that is based in Dubai, committed to the running of a laboratory for the banking and use of all sorts of allogeneic and autologous cellular products. Through the proliferation of cellular therapies throughout the world, Bioscience Clinic hopes to advance treatment options for some debilitating diseases that current medical science has been largely unable to target the root causes of.

About Global Stem Cells Group
Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations, each focused on furthering scientific and technological advancements to lead cutting-edge stem cell development, treatments, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop hub for stem cell solutions that adhere to the highest medical standards.
- Published in Press Releases
Global Stem Cells Group Announces Training in Cochabamba, Bolivia at the End of November
The Global Stem Cells Group, a multi-disciplinary community of scientists and physicians that are collaborating to treat diseases and lessen human suffering through the advancement of the field of regenerative medicine has announced that the construction of their Stem Cell Center in Cochabamba, Bolivia, has concluded. What’s more, the organization has announced that, in celebration of the facility’s completion, the Center’s inaugural training course will take place at the end of November.
The new facility located in Bolivia is the 35th Stem Cell Center in the world, strengthening the Global Stem Cells Group’s presence worldwide as they seek to expand research for and the
practice of regenerative medicine across the globe. Representatives from the Group and local physicians in the area will both see the impact of this new clinic, which will offer a permanent space within the country where experts in the field can train Bolivian physicians in the latest stem cell research.
“I am very excited for the opportunity to train more Bolivian physicians. We’ve been laying the groundwork for this Stem Cell Center for a very long time, and it feels almost like a dream to have to ready to unveil to the world by the end of November,” Said Benito Novas, founder and CEO of the Global Stem Cells Group, “To be able to have a permanent location in Cochabamba, and to have spots quickly filling up for this training– I think it speaks volumes to the future of regenerative medicine being a bright one,”
This inaugural training is intended to not only teach physicians the value of incorporating regenerative medicine into their own clinics, but to ensure that there is a vast store of all the necessary equipment and supplies that are required for the wide array of cellular therapies that are available for patients around the world– including a highly interactive study session that goes over the extraction, isolation, and application of PRP, Adipose, ad Bone Marrow Stem Cells.
The Center will also provide access to several texts detailing procedure processes and treatment options that are available for reference after the training is completed.
If you are interested in learning more about the Global Stem Cell Group’s Onsite Regenerative Medicine Training, or to book your seat, you can visit us at our training website
About Global Stem Cells Group
Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations, each focused on furthering scientific and technological advancements to lead cutting-edge stem cell development, treatments, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop hub for stem cell solutions that adhere to the highest medical standards.
- Published in Press Releases
Global Stem Cells Group CEO Benito Novas Confirmed Speaker at 1st Ibero-American Fellowship Program in Regenerative and Functional Medicine
The First International Iber-American University Diploma has been announced to focus on laser therapy, hormone therapy, and wellness, and brings with it over a dozen seasoned instructors. The fellowship program, which spans over multiple weeks, covers a variety of topics with the focus of expanding the professional vision of medical professionals, and furthering knowledge of the applications of lasers in gynecology and cosmetic medicine. Additionally, it will update physicians on the newest techniques developed around the world to improve the quality of life of patients. A variety of protocols will be covered, including office, operating room, and hormone-based procedures.
The Fellowship Program has been certified by Atlantic International University, which is a private university based in Honolulu, Hawaii. Those physicians who complete the fellowship will be asked to demonstrate their knowledge through the use of clinical case studies involving real patients. Additionally, AIU offers students the opportunity to supplement this program with resources in various areas of knowledge, at no extra cost.
Benito Novas, CEO of Global Stem Cells Group, had this to say about the conference: “I am extremely proud and excited to be bringing this conference to physicians around the world. The program has been organized and directed by dear friends of mine, who have put in countless hours to ensure over a dozen physicians from a wide variety of specialties– really, nearly every field of medicine will be represented, and the course has been structured to ensure maximum retention for each and every student of ours,”
As a result, Global Stem Cells Group and the International Society for Stem Cells Application have both officially endorsed the course as a way for a physician to increase his knowledge in subjects ranging from cosmetic surgery, to marketing strategies, to hormone therapy, and everything in between. To learn more about the First International Ibero-American University Diploma, you may send an email to aiu-curso@amgerweb.com.
About Global Stem Cells Group
Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations, each focused on furthering scientific and technological advancements to lead cutting-edge stem cell development, treatments, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop hub for stem cell solutions that adhere to the highest medical standards.

- Published in Press Releases