The FDA Vs Stem Cell Treatments In The US
Introduction
Stem cell therapy has become a popular type of treatment for a number of conditions, including the regeneration of injured tissue or diseased cells in the human body. This therapy is classified as regenerative medicine. Several advancements have already been made in this medical field, and many have experienced positive results when they underwent stem cell therapy to assist in the treatment of degenerative conditions.
Benefits and Concerns of Stem Cell Therapy
While stem cell therapy has been proven to provide an effective protocol in the recovery of stroke, neurological issues, and even to assist in repairing the damage caused by a heart attack, recent reports have caused many to view these treatments negatively. This is due to warnings that were recently issued by the FDA. Even though some have experienced side effects due to the use of stem cell therapy, it is important to note that there are facilities within the US that can provide professional services that help to minimize the risk of these adverse events.
The FDA’s Stance on Stem Cell Therapy in the United States
The Food and Drug Administration of the United States is involved in analyzing and approving medications and medical procedures that may be offered to patients in the country. To date, the FDA has shown some interest in stem cell therapy, providing approval for several clinical trials that looked at how effective this treatment is in providing a regenerating effect on the impact that certain conditions have on the human body. However, only one particular treatment involving stem cell therapy has been officially approved by the FDA.
Recent FDA Actions and Their Impact
Within the last few months, several concerning publications have been made by the FDA regarding stem cell therapies, reducing the hope that some patients might have gained. On June 25, 2019, the FDA published a statement describing a permanent injunction against many stem cell clinics in the country. The FDA stated that the action taken from their side is to assist in providing a layer of protection for patients, due to the risks involved with undergoing treatments that utilize products that have not gone through any type of approval process.
The Case Against US Stem Cell Clinic LLC
The specific company targeted in this statement and by the actions taken by the FDA is known as US Stem Cell Clinic LLC. The court ruled in favor of the FDA and US government earlier in June, finding the defendants, being US Stem Cell Clinic LLC, guilty of the claims made against them. An earlier publication also confirmed that the Federal court ruled the misbranding of the stem cell products released by the US Stem Cell Clinics company as a violation of laws implemented to protect the people of the country.
Implications of FDA Regulations on Stem Cell Treatments
News publications have announced that the regulations and guidelines set out by the FDA might cause a significant decline in the availability of stem cell treatments to patients in the United States. The companies targeted by these particular actions are primarily those marketing products that have not gone through approval phases – and these products have been found to put the patient’s health at risk.
The Role of Stem Cell Therapy in Disease Treatment
While some companies have been found guilty of using unapproved and misleading products on patients, promoted as stem cell therapy, it is important to consider the reality of the situation as well. Stem cell therapy, often referred to simply as cell therapy, has been proven to be a successful regime in the treatment of several conditions. Many of the conditions that have shown improvement with this therapy were previously considered difficult, or sometimes even impossible, to treat effectively.
Global Success of Stem Cell Therapy
By 2012, the Worldwide Network for Blood and Marrow Transplantation (WBMT) announced that a total of one million stem cell therapy procedures had been done throughout the world. This was a significant milestone, and these procedures have helped to save countless lives. The WBMT refers to a case of Marta, a girl from Madrid, who received stem cell therapy at a young age after being diagnosed with Leukemia. In 2002, Marta was able to overcome Leukemia, thanks to the cell therapy provided to her.
Expanding Applications of Stem Cell Therapy
While lymphoma and leukemia are the conditions where patients most frequently seek stem cell therapy, many other conditions are treated with this procedure today. The perfection of the therapy has led to treatments that can assist in reducing the effects of over 70 diseases that may otherwise have a significant impact on the human body.
GSCG Expands to Cancun, Meets the Demands of US Patients
The Global Stem Cells Group recently announced the opening of a new office in Cancun, Mexico. This decision was driven by the increasing demand for cell therapy procedures by patients in the United States and the lack of facilities able to provide these individuals with professional and quality services. Doctors specializing in regenerative medicine are also having a hard time fulfilling the needs of patients who wish to consider cell therapy as a potential treatment option.
GSCG’s Facilities in Cancun
This is not the only local office that GSCG operates in Cancun, as the company now has two facilities within this location. These offices include a stem cells laboratory and a general medical facility. Over the years, GSCG has become an established name in the stem cell field, providing thousands of doctors the opportunity to offer cell therapy as a treatment option for patients with degenerative diseases. This therapy may assist in the regeneration of damaged and diseased tissues in the patient’s body.
Ensuring Quality and Safety at GSCG
Even though a previous office had established a presence for GSCG in Cancun, the company announced that the opening of the new office means they have now officially created a permanent presence for the brand in the area. GSCG’s new facility in Cancun is equipped with the latest advancements in the field and provides quality services that ensure patients have a trusted medical facility where they can undergo cell therapy as part of a regenerative medicine treatment plan.
Support for US Doctors in Regenerative Medicine
Doctors in the field of regenerative medicine within the United States can now turn to this facility to offer their patients an additional treatment option, apart from the standard pharmaceutical protocols. The stem cell laboratory can assist in analyzing patient data and finding a matching donor for those in need of stem cell treatment. GSCG’s facility can help with the culturing of expanded autonomous cells and other cell types utilized in stem cell therapy. A detailed approach is taken, considering the age of the donor, which provides an overview of the autonomous stem cell age. The patient being treated is also closely considered, as there are different methods of introducing these stem cells to the body for enhanced efficacy.
Advanced Treatment Methods at GSCG
A more aggressive method is often needed for patients with conditions such as autism, Alzheimer’s disease, and kidney-related conditions. Other conditions treated include pulmonary diseases and Parkinson’s disease. In cases of stroke, the vascular arteries, along with the carotid artery, are targeted with the therapy for a more effective approach to assisting with the regeneration of damaged structures in the patient’s brain. This ultimately leads to a significant improvement in the delivery of stem cells to the patient’s body, enhancing the overall efficacy of the treatment.
Comprehensive Patient Care at GSCG
When procedures are provided to the patient through the GSCG, an appropriate ICU unit is made available to ensure the patient is provided with adequate care during the operation and recovery. The utilization of recent technological advancements at GSCG laboratories can also assist in providing a more economical approach to cell therapy. Allogenic stem cell procedures can be addressed using techniques that reduce the costs involved, making these treatments available to patients at a lower price.
Advanced Cultivation Techniques at GSCG
The utilization of autologous stem cell transplantation offers an opportunity for patients to undergo stem cell therapy when a sibling does not provide an ideal match. Cells can be cultivated from the bone marrow and treated with specific drugs to yield targeted results. In the case of malignant cells, bone marrow can be treated with monoclonal antibodies or cytotoxic drugs to target such cells when transplanted back into the patient. Appropriate storage solutions are also considered to assist with the cultivation process and reduce the risk of enzymatic treatment leading to the differentiation of cells into other types. The culture condition becomes crucial for the expansion of stem cells, utilizing mediums and techniques that ensure successful cultivation and expansion.
The GSCG’s Role in the New FDA Warnings
The FDA’s recent warnings and actions against certain stem cell therapy products have caused a limitation in access to these treatments. With the seizure of services provided by companies affected by the FDA’s actions, patients may not be sure where to turn. This issue also affects many doctors in regenerative treatments and medicine, as they need to ensure that the facilities they use for patients offer safe procedures.
GSCG’s Trusted Services
This is where the GSCG comes into the picture. With an established presence in Mexico, doctors now have access to a facility with a history of providing quality stem cell therapy services to patients with qualifying diseases. The company is trusted and has performed stem cell therapy procedures that have led to successful results.
Hope for Patients Through GSCG
These treatments may help repair neurological problems caused by disorders like Parkinson’s disease, stroke, or spinal cord injuries. They may also offer new hope to people with diabetes, heart disease, and those who have suffered cardiovascular damage due to a heart attack.
Conclusion
Even though recent FDA publications have raised concerns regarding stem cell therapy, many patients are still interested in undergoing these treatment procedures. A significant number of studies have provided evidence on the efficacy of treating tissue damage caused by various conditions through stem cell therapy. Patients interested in undergoing stem cell treatment are now advised to turn their interest to Stem Cells Centers in Cancun and related regions, where highly specialized and experienced doctors can provide professional service.
- Published in Corporate News / Blog
How To Effectively Treat Amyotrophic Lateral Sclerosis (ALS)
What is ALS?
Although a rare neurologic condition, Amyotrophic Lateral Sclerosis (ALS) is the most common type of Motor Neuron Disease (MND), a condition that affects the voluntary muscles. This is a progressive disorder that leads to muscle weakness and depletion due to nerve dysfunction.
ALS is also called Lou Gehrig’s disease, named after the football player who had this condition. The literal meaning of Amyotrophic is ‘no muscle nourishment,’ which becomes the cause of muscle atrophy. ‘Lateral’ refers to the group of nerves in the spinal cord that sends signals to the muscles. It is these nerves that degenerate, leading to sclerosis in this region. In later stages, this affects the nerves that control breathing and hence can be fatal.
Initial Symptoms of ALS
The initial symptoms of ALS include stiffness and muscle weakness, which gradually involve all the muscles under voluntary control. The affected regions and progressive pattern vary from one person to another. Some have difficulty holding a pen or a cup, while others find difficulty speaking, chewing, or even talking. Thus, ALS is an ailment that affects daily life and makes simple tasks painful and troublesome.
ALS Statistics
According to the Center for Disease Control and Prevention (CDC), 14,500 to 15,000 people had ALS in the United States in 2016, with approximately 5000 people having a confirmed diagnosis for the condition annually. Although the average survival rate is three to five years, patients can live for ten years or more.
Types of ALS
Sporadic ALS
This is the most common type and affects 95% of sufferers. This type occurs without a clear cause.
Familial ALS (FALS)
This type occurs in 5-10% of sufferers. This type of ALS is genetic and runs in families. This occurs due to abnormal changes to a gene that is then passed in generations.
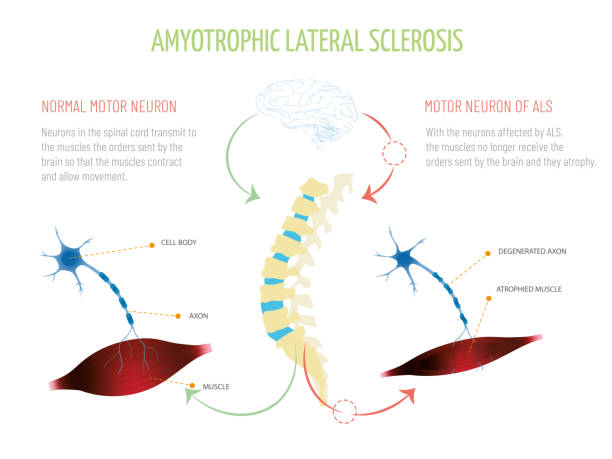
Symptoms of ALS
What are the symptoms of ALS?
Early signs and symptoms might be unnoticeable and become perceptible after some time. Most clinical signs are evident of upper motor neuron and lower motor neuron lesions. The limb onset ALS (70%) involves initial symptoms in the limbs, while the bulbar onset ALS (25%) is characterized by speech and swallowing problems, followed by weakness in the limbs later. The remaining 5% of patients have respiratory involvement in the early period.
Most Common Symptoms Include:
- Muscle weakness in the limbs (distal or proximal)
- Asymmetric progressive muscle wasting
- Difficulty in motor activities like walking, talking, chewing
- Weakness in arms, legs, hands, and feet
- Muscle cramps and twitching
- Slurred speech
- Fatigue
- Emotional liability (episodes of uncontrolled laughing and crying)
- Difficulty in maintaining posture and gait
- Difficulty in breathing and swallowing
With the progression of the disease, symptoms may spread to all parts of the body. In some patients, frontotemporal dementia may occur, resulting in poor memory and decision-making abilities.
Causes of ALS
The exact cause of ALS has not been known by scientists to date. However, research is being carried out to understand what causes ALS. There are several different factors such as:
Genetic Changes
Studies have shown that 5 to 10% of cases of ALS are caused by genetic mutations. For example, changes to the gene that makes SOD1 protein causes damage to motor neurons.
Environmental Factors
No major association has been established between environmental factors like toxins, viruses, diet, or physical trauma and the risk of development of ALS. However, there is ongoing research on the subject. Studies have shown that some athletes are at a higher risk of acquiring ALS due to vigorous physical activity.
Chemical Disturbance
Glutamate is the neurotransmitter that is in control of signals to and from the brain. Accumulation of this neurotransmitter within the spaces surrounding the nerves damages them. Research has also shown mitochondrial structural and functional abnormalities, as well as defects in axonal structure and transport, could be the causative agents for ALS.
Diagnosis of ALS
When it comes to diagnosis, there are no specific tests that can provide a definitive diagnosis for ALS. However, doctors conduct a series of tests to rule other similar diseases. A full medical history check and a neurologic examination are undertaken at regular intervals to assess the progressive worsening of symptoms.
Diagnostic Tests Include:
- Electromyography (EMG): EMG records the electrical activity of the muscle fibers.
- Nerve Conduction Study (NCS): NCS assesses the electrical activity of the nerves and muscles.
- Magnetic Resonance Imaging (MRI): MRI rules out other possible conditions such as a tumor or cyst in the spinal cord, cervical spondylosis, or a hernia in the neck that could be causing the nerve compression.
Laboratory tests such as blood screening and urine tests can also be carried out so that other diseases can be eliminated.
Treatment Options & Management Strategies for ALS
ALS is managed through a multidisciplinary approach. Unfortunately, there is no definitive cure for the disease at this time. Management of ALS is done through symptomatic treatment to ease the condition of the patients and prevent unnecessary complications:
Support
Physicians, psychologists, speech therapists, nutritionists, and home care assistance all play a vital role in making life easier for patients with ALS.
Medication
Riluzole (Rilutek) and Edaravone (Radicava) are the drugs approved by the U.S Food and Drug Administration (FDA) for treating ALS. Riluzole is believed to reduce glutamate levels, thereby decreasing damage to the motor neurons. Edaravone acts as an antioxidant and is believed to expel free radicals and reduce oxidative stress in the motor neurons.
Lifestyle Habits
Physiotherapists can recommend exercise and physical activity like walking, swimming, and bicycling that may improve muscle strength and help elevate mood without overstressing the muscles.
Speech Therapy
Therapists can help patients with ALS to employ strategies to speak clearly. They may also recommend computerized aids such as speech synthesizers and eye-tracking technology to help people learn ways for responding by nonverbal means.
Diet
Nutritionists may formulate a diet plan for patients, which consists of food that is easy to swallow and provides enough nourishment and calories for the patients to maintain adequate energy levels and to prevent excessive weight loss.
Breathing Support
Patients with ALS may suffer from shortness of breath and difficulty breathing during physical activity or while lying down. If this is the case, doctors can recommend Non-Invasive Ventilation (NIV) that provides breathing support through the nose or mouth. NIV improves quality of life and increases the number of years of survival for patients.
Is Stem Cell Therapy an Option?
As previously mentioned, there is no curable treatment for ALS available. However, scientists are researching Stem Cell Therapy as the new favorable approach in the treatment of neurologic disorders. There is a rising interest in Stem Cell Therapy as a promising remedy for curing ALS. Mesenchymal stem cells are particularly believed to be the most suitable ones due to their availability, absence of ethical issues, and positive results in various experiments.
Research on Stem Cell Therapy
Studies and clinical trials have begun to apprehend the benefits of MSC transplantation. They demonstrate that MSCs lead to a partial recovery of motor neurons and a delay in disease progression. Also, there has been no evidence of a major adverse effect after MSC transplantation. When testing this newfound research on animals, the lifespan of the subjected animal has increased with MSC transplantation. These positive results have encouraged the administration of MSC in ALS patients.
However, despite the safe outcomes of MSC transplantation in humans, results show that there is only a partial improvement in ALS sufferers with only a few cases that showed a delay in disease progression. Hence, there is a need for further studies and trials on a higher number of human subjects for a better understanding of MSC effects so that more significant conclusions can be reached.
- Published in Corporate News / Blog
Which Works Better, Viscosupplementation, or Platelet Rich Plasma?
Introduction to Viscosupplementation
One of the treatments approved by the FDA for knee arthritis is Hyaluronic Acid (HA) injection, commonly known as viscosupplementation. This treatment has shown significant success in managing knee arthritis and is increasingly being explored for other parts of the body, such as the hip and shoulder, although these uses are not FDA-approved. HA injections mimic the fluid that naturally surrounds your joints, acting as a lubricant and shock absorber, thereby reducing arthritis pain. Over time, HA is absorbed into the joint, potentially stimulating the body to produce more stable cartilage.
Evidence Supporting Viscosupplementation
The evidence supporting HA injections is robust. A systematic review of 76 randomized controlled trials concluded that HA injections can improve function, reduce pain, and serve as a reliable treatment for knee osteoarthritis.
The Role of Platelet-Rich Plasma (PRP) in Treating Arthritis
What is Platelet-Rich Plasma (PRP)?
Platelet-Rich Plasma (PRP) is a concentrate of platelets in plasma, containing 6 to 10 times more platelets than normal blood. PRP is rich in growth factors, such as Epidermal Growth Factor (EGF) and Connective Tissue Growth Factor (CTGF), which promote healing by leveraging the body’s natural healing mechanisms.
FDA Regulation and Research on PRP
Unlike HA, PRP is not FDA-regulated, and the devices used to prepare PRP are subject to FDA approval. Despite this, numerous studies have demonstrated PRP’s efficacy in treating tendon injuries and osteoarthritis, as well as in reducing pain. Ongoing research is exploring its potential benefits for hair regrowth, cardiac muscle repair, and dermatologic rejuvenation.
Comparing HA Injections and PRP
Effectiveness and Safety
Studies indicate that PRP can be just as effective, if not more so, than HA injections. While HA is FDA-approved only for knee use, PRP is derived from the patient’s own blood, reducing the risk of infection and autoimmune reactions, thanks to the presence of white blood cells that help fight infections.
Cost Considerations
HA injections for joints other than the knee are not FDA-approved or typically covered by insurance, often costing patients $1500 or more, excluding additional charges like doctor visits and the injection itself. In contrast, PRP treatments generally cost between $800 and $1200 out of pocket.
Conclusion: Which is the Better Choice?
PRP has demonstrated comparable or superior effectiveness to HA injections for arthritis pain management. It carries a lower risk of infection or autoimmune reactions and is generally more cost-effective. Given these advantages, PRP often emerges as the preferred choice for many patients and healthcare providers.
- Published in Corporate News / Blog
Platelet-Rich Plasma Injections: Protocol Guide
Almost all sports medicine doctors agree that there’s no harm in trying Platelet Rich Plasma Injections (PRP Injections) for their patients. After all, there are hundreds of thousands of cases with positive results. All it needs is research to prove its worth. Currently, many independent researches are ongoing, funded privately, like the one conducted by Dr. Kimberly G. Harmon M.D., director of the Primary Care Sports Medicine fellowship at the University of Washington. She recently received a gift to support her research from UW alumni who firmly believe in Platelet-Rich Plasma (PRP).
While the process of extracting PRP is fairly simple—there are many variants as long as platelets are above baseline levels with at least seven growth factors—many physicians are still unsure about what they can and can’t do when it comes to this marvelous procedure. Today, let’s shed light on the fine print.
Platelet-Rich Plasma Injections Protocol
Protocol/Technique
Usually, the procedure requires the physician/surgeon and an assistant or two to help with the preparation of the graft, maintenance of sterile technique, and saving the ultrasound images (if relevant).
Pre-Procedure Considerations
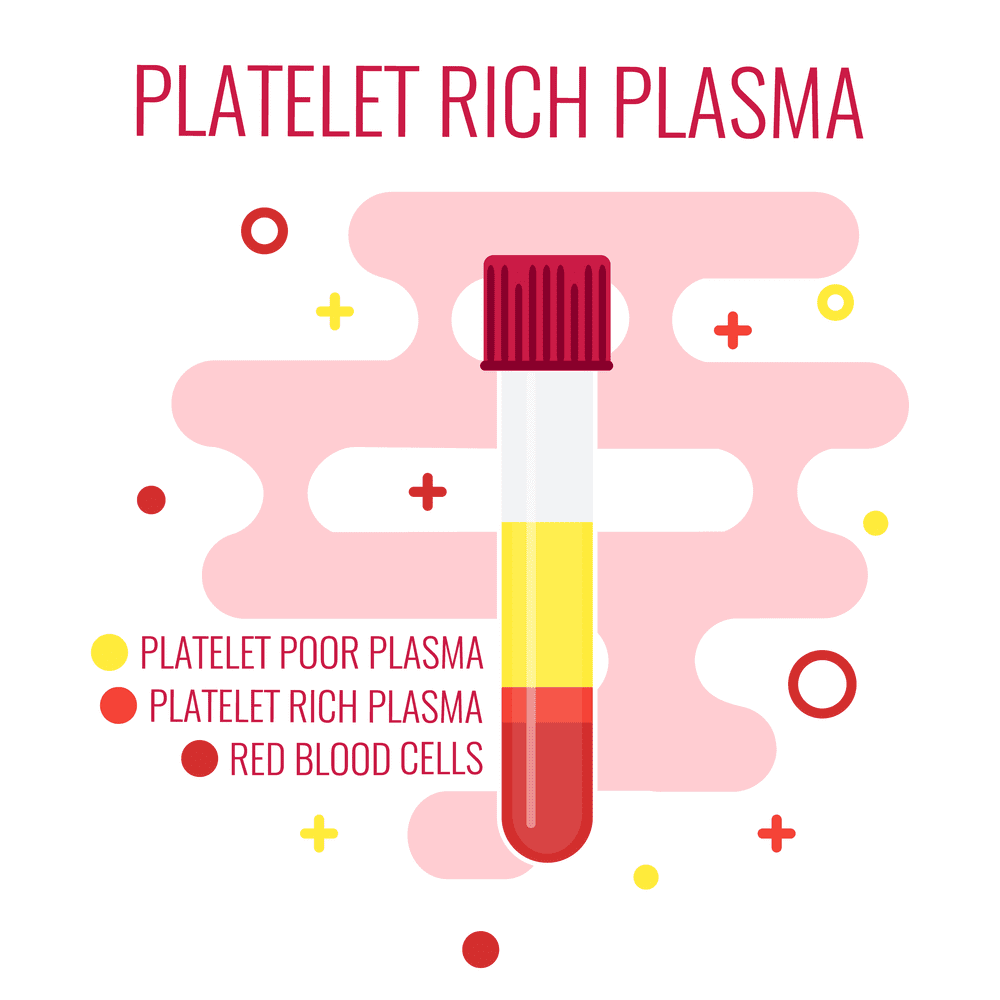
- There should always be a specific indication associated with a physical exam with confirmed imaging studies such as an ultrasound, CT scan, or MRI before treatment.
- Proper patient education and a discussion must be had with the patient, as well as a signed informed consent prior to the procedure.
- Contraindications must be reviewed prior to the procedure.
Graft Preparation
- The patient should be positioned in a comfortable seated or reclining position.
- Sterile single needles and syringes must be used with proper handling and disposal.
- Using an aseptic procedure, the proper amount of blood is then drawn from the vein for the PRP procedure.
- If the blood cannot be obtained from the site the first time, a new site must be used to prevent early activation.
- Using a sterile technique, transfer the tube of venous blood to the centrifuge. Platelet-Rich Plasma should be acquired using a separating device created for autologous blood. Preference is always given to a closed system that will prevent exposure of the blood and its cellular components to the open air, and permits minimal use of the tissue.
Image Guidance PRP Therapy
- Real-time imaging guidance using ultrasound, CT, or fluoroscopy should always be used when performing a PRP injection.
- If ultrasound is going to be used, the following considerations need to be decided in advance: For lengthy procedures, PRP injections near the spine, and intra-articular injections, sterile gel is recommended.
- Always use sterile probe covers. Cleansing the probe before and after the PRP procedures and observing sterile technique is sufficient.
- Guided images and indelible markings of the site of the probe position and the needle entry always need to be made before cleaning the skin where the probe and needle will be inserted.
- Always apply a bandage or a dressing after the procedure to protect the entry site from germs.
Post-Injection Care
- The patient should be monitored for any post-PRP procedure complications such as vaso-vagal reactions.
- Patients should be given post-procedure directions and precautions, and any questions should be answered before they leave. They should also have emergency contact information.
- Patients should be instructed about immobilization and any post-procedure activities that are allowed and/or not allowed.
- Post-PRP procedure pain prescriptions need to be given to the patient before discharge, and any questions about the medication(s) should be answered at this time. Patients also need to be instructed to avoid NSAIDs until they have healed, are pain-free, and have full function returned to the treated area.
- Per OSHA guidelines, contaminated areas must be disinfected before the next patient uses the room.
- The PRP procedure must be documented in detail, including a procedure note with the following information: date, pre- and post-procedure diagnosis, name of the procedure, physician/surgeon(s), any assistants, whether or not anesthesia was used and what type, short-term indication of the procedure, a description of the graft preparation, and a description of the procedure, including any/all guidance and instruments used.
Follow-up Care
- Patients are normally re-examined 2-6 weeks after the PRP procedure to follow up on pain, use, the injection site, and to discuss any concerns and future course of action.
- The patient response to the treatment should be recorded using authenticated outcome measures.
- Any complications, responses, and all other relevant information should be logged into the ICMS tracking system.
- Consideration for another PRP injection should be the center of the discussion, and the patient will be able to make a decision based on the outcome.
Safety Considerations
- Universal precautions must be used before, during, and after the procedure.
- Risk of infection: PRP is antimicrobial and provides effective protection against most bacterial infections except for Klebsiella, Pseudomonas, and Enterococcus.
- With the graft being made entirely out of autologous blood, it virtually eliminates the risk of disease transmission unless the graft becomes contaminated.
Risks to Patients from the Procedure
- Infection
- Bleeding
- Nerve damage
- Pain
- Lack of result
- Loss of limb and death (very rare)
Platelet-Rich Plasma: Indications
Musculoskeletal complaints require a complete history and exam to find a diagnosis. Often, diagnostic studies may be needed and reviewed to understand why prior treatments failed. PRP is usually considered an optional treatment for chronic and subacute conditions. Commonly, healing slows down or stops altogether at the 6-12 week period following an acute or traumatic injury. If the patient has not had any improvement for over the first six weeks, it’s probable the healing period has stopped.
Platelet-Rich Plasma: Contraindications
- Septicemia
- Platelet dysfunction syndrome
- Localized infection at the procedure site
- Hemodynamic instability
- Critical thrombocytopenia
- Patient not willing to take the risks involved with the procedure
Relative Contraindications:
- Regular use of NSAIDs within 48 hours of the PRP procedure
- HGB of < 10 g/dl
- Platelet count of < 105/ul
- Systemic use of corticosteroids within 2 weeks
- Recent illness or fever
- Cancer, particularly hematopoietic or bone
- HGB < 10 g/dl
- Platelet count < 105/ul
- Corticosteroid injection at the treatment site within 1 month
- Tobacco use
- Published in Corporate News / Blog
Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise
Introduction
The clinical demand for bioengineered blood vessels continues to rise, yet current options for vascular conduits remain limited. The synergistic combination of emerging advances in tissue fabrication and stem cell engineering promises new strategies for engineering autologous blood vessels that recapitulate not only the mechanical properties of native vessels but also their biological function. Here, we explore recent bioengineering advances in creating functional blood macro and microvessels, particularly featuring stem cells as a seed source. We also highlight progress in integrating engineered vascular tissues with the host after implantation and the exciting pre-clinical and clinical applications of this technology.
The Clinical Need for Vascular Grafts
Ischemic diseases, such as atherosclerotic cardiovascular disease (CVD), remain one of the leading causes of mortality and morbidity worldwide (GBD 2015 Mortality and Causes of Death Collaborators, 2016; Mozaffarian et al., 2016). These diseases have resulted in an ever-persistent demand for vascular conduits to reconstruct or bypass vascular occlusions and aneurysms. Synthetic grafts for replacing occluded arterial vessels were first introduced in the 1950s following surgical complications associated with harvesting vessels, the frequent shortage of allogeneic grafts, and immunologic rejection of large animal-derived vessels. Despite advances in pharmacology, materials science, and device fabrication, these synthetic vascular grafts have not significantly decreased overall mortality and morbidity (Nugent and Edelman, 2003; Prabhakaran et al., 2017).
Challenges with Synthetic Grafts
Synthetic grafts continue to exhibit a number of shortcomings that have limited their impact. These shortcomings include low patency rates for small diameter vessels (< 6 mm in diameter), a lack of growth potential for the pediatric population necessitating repeated interventions, and susceptibility to infection. Vascular conduits are also needed for clinical situations such as hemodialysis, which involves large volumes of blood that must be withdrawn and circulated back into a patient several times a week for several hours.
The Importance of Microvascularization
In addition to large-scale vessel complications, ischemic diseases also arise at the microvasculature level (< 1 mm in diameter), where replacing upstream arteries would not address the reperfusion needs of downstream tissues (Hausenloy and Yellon, 2013; Krug et al., 1966). Microvascularization has proven to be a critical step during regeneration and wound healing, where the delay of wound perfusion (in diabetic patients, for example) significantly slows down the formation of granulation tissue and can lead to severe infection and ulceration (Baltzis et al., 2014; Brem and Tomic-Canic, 2007; Randeria et al., 2015).
Structural Components of Blood Vessels
To design advanced grafts, it is important to consider the structural components of a blood vessel. Many different blood vessel beds share common structural features. Arteries, veins, and capillaries have a tunica intima comprised of endothelial cells (EC), which regulate coagulation, confer selective permeability, and participate in immune cell trafficking (Herbert and Stainier, 2011; Potente et al., 2011). Arteries and veins are further bound by a second layer, the tunica media, composed of smooth muscle cells (SMC), collagen, elastin, and proteoglycans, conferring strength to the vessel and acting as effectors of vascular tone. Vascular tissue engineering has evolved to generate constructs that incorporate the functionality of these structural layers, withstand physiological stresses inherent to the cardiovascular system, and promote integration in host tissue without mounting immunologic rejection (Chang and Niklason, 2017).
Suitable Cell Sources
A suitable cell source is critical to help impart structural stability and facilitate in vivo integration. Patient-derived autologous cells are one potential source that has garnered interest because of their potential to minimize graft rejection. However, isolating and expanding viable primary cells to a therapeutically relevant scale may be limited, given that patients with advanced arterial disease likely have cells with reduced growth or regenerative potential. With the advancement of stem cell (SC) technology and gene editing tools such as CRISPR, autologous adult and induced pluripotent stem cells (iPSCs) are emerging as promising alternative sources of ECs and perivascular SMCs that can be incorporated into the engineered vasculature (Chan et al., 2017; Wang et al., 2017).
Engineering and Integration of Vascular Tissues
A viable cell source alone is not sufficient for therapeutic efficacy. Although vascular cells can contribute paracrine factors and have regenerative capacity, merely delivering a dispersed mixture of ECs to the host tissue has shown limited success at forming vasculature or integrating with the host vasculature (Chen et al., 2010). Recent tissue engineering efforts have focused on recreating the architecture and function of the vasculature in vitro before implantation, with the hypothesis that pre-vascularized grafts and tissues enhance integration with the host.
Clinical Applications
The first reported successful clinical application of tissue-engineered blood vessels (TEBV) in patients was performed by Shin’oka et al., who implanted a biodegradable construct as a pulmonary conduit in a child with pulmonary atresia and single ventricle anatomy (Shin’oka et al., 2001). The construct was composed of a synthetic polymer mixture of L-lactide and e-caprolactone, reinforced with PGA, and seeded with autologous bone marrow-derived mesenchymal stem cells (BM-MSCs). The authors demonstrated patency and patient survival 7 months post-implant, and expanded their study to a series of 23 implanted TEBVs and 19 tissue patch repairs in pediatric patients (Hibino et al., 2010). They reported no graft-related mortality, and four patients required interventions to relieve stenosis at a mean follow-up of 5.8 years.
The first sheet-based technology to seed cultured autologous cells, developed by L’Heureux et al., induced cultured fibroblast cell sheets over a 10-week maturation period to produce tubules of endogenous ECM over a production time ranging between 6 and 9 months. They dehydrated and provided a living adventitial layer before seeding the constructs with ECs (L’Heureux et al., 2006). Their TEBV, named the Lifeline graft, was implanted in 9 of 10 enrolled patients with end-stage renal disease on hemodialysis and failing access grafts in a clinical trial. Six of the nine surviving patients had patent grafts at 6 months, while the remaining grafts failed due to thrombosis, rejection, and failure (McAllister et al., 2009).
Future Directions
Harnessing the regenerative functions reported in ECs derived from adult stem cells and iPSCs offers the promise of improving TEBV patency. Mcllhenny et al. generated ECs from adipose-derived stromal cells, transfected them with an adenoviral vector carrying the endothelial nitric oxide synthase (eNOS) gene, and seeded the ECs onto decellularized human saphenous vein scaffolds (McIlhenny et al., 2015). They hypothesized that through inhibition of platelet aggregation and adhesion molecule expression, nitric oxide synthesis would prevent thrombotic occlusion in TEBV. Indeed, they reported patency with a non-thrombogenic surface 2 months post-implantation in rabbit aortas. Engineering ECs and SMCs with other regenerative, anti-inflammatory, and anti-thrombotic genes could bridge the functional difference between SC-derived cells and native primary cells.
- Published in Corporate News / Blog





