The FDA Vs Stem Cell Treatments In The US
Introduction
Stem cell therapy has become a popular type of treatment for a number of conditions, including the regeneration of injured tissue or diseased cells in the human body. This therapy is classified as regenerative medicine. Several advancements have already been made in this medical field, and many have experienced positive results when they underwent stem cell therapy to assist in the treatment of degenerative conditions.
Benefits and Concerns of Stem Cell Therapy
While stem cell therapy has been proven to provide an effective protocol in the recovery of stroke, neurological issues, and even to assist in repairing the damage caused by a heart attack, recent reports have caused many to view these treatments negatively. This is due to warnings that were recently issued by the FDA. Even though some have experienced side effects due to the use of stem cell therapy, it is important to note that there are facilities within the US that can provide professional services that help to minimize the risk of these adverse events.
The FDA’s Stance on Stem Cell Therapy in the United States
The Food and Drug Administration of the United States is involved in analyzing and approving medications and medical procedures that may be offered to patients in the country. To date, the FDA has shown some interest in stem cell therapy, providing approval for several clinical trials that looked at how effective this treatment is in providing a regenerating effect on the impact that certain conditions have on the human body. However, only one particular treatment involving stem cell therapy has been officially approved by the FDA.
Recent FDA Actions and Their Impact
Within the last few months, several concerning publications have been made by the FDA regarding stem cell therapies, reducing the hope that some patients might have gained. On June 25, 2019, the FDA published a statement describing a permanent injunction against many stem cell clinics in the country. The FDA stated that the action taken from their side is to assist in providing a layer of protection for patients, due to the risks involved with undergoing treatments that utilize products that have not gone through any type of approval process.
The Case Against US Stem Cell Clinic LLC
The specific company targeted in this statement and by the actions taken by the FDA is known as US Stem Cell Clinic LLC. The court ruled in favor of the FDA and US government earlier in June, finding the defendants, being US Stem Cell Clinic LLC, guilty of the claims made against them. An earlier publication also confirmed that the Federal court ruled the misbranding of the stem cell products released by the US Stem Cell Clinics company as a violation of laws implemented to protect the people of the country.
Implications of FDA Regulations on Stem Cell Treatments
News publications have announced that the regulations and guidelines set out by the FDA might cause a significant decline in the availability of stem cell treatments to patients in the United States. The companies targeted by these particular actions are primarily those marketing products that have not gone through approval phases – and these products have been found to put the patient’s health at risk.
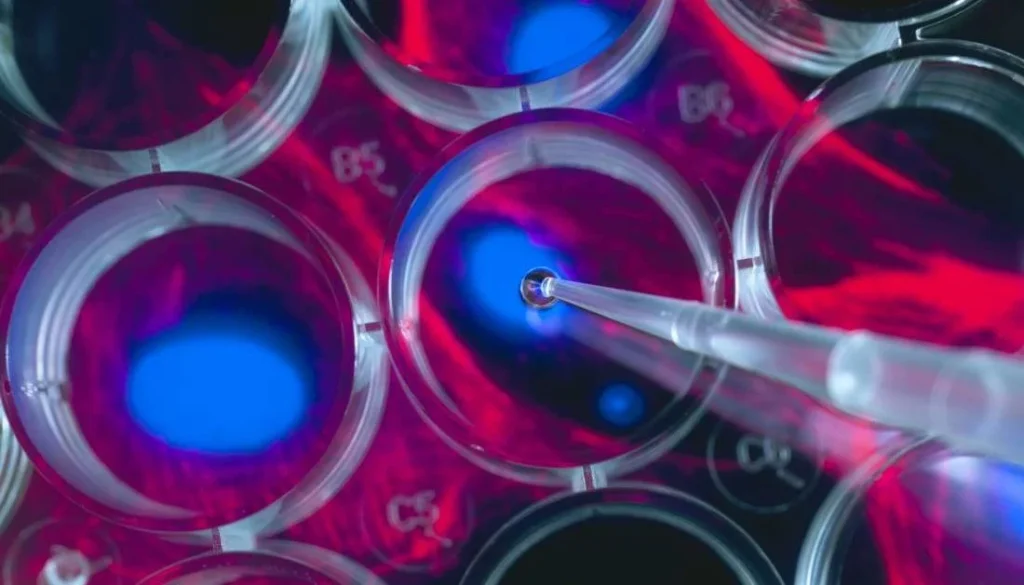
The Role of Stem Cell Therapy in Disease Treatment
While some companies have been found guilty of using unapproved and misleading products on patients, promoted as stem cell therapy, it is important to consider the reality of the situation as well. Stem cell therapy, often referred to simply as cell therapy, has been proven to be a successful regime in the treatment of several conditions. Many of the conditions that have shown improvement with this therapy were previously considered difficult, or sometimes even impossible, to treat effectively.
Global Success of Stem Cell Therapy
By 2012, the Worldwide Network for Blood and Marrow Transplantation (WBMT) announced that a total of one million stem cell therapy procedures had been done throughout the world. This was a significant milestone, and these procedures have helped to save countless lives. The WBMT refers to a case of Marta, a girl from Madrid, who received stem cell therapy at a young age after being diagnosed with Leukemia. In 2002, Marta was able to overcome Leukemia, thanks to the cell therapy provided to her.
Expanding Applications of Stem Cell Therapy
While lymphoma and leukemia are the conditions where patients most frequently seek stem cell therapy, many other conditions are treated with this procedure today. The perfection of the therapy has led to treatments that can assist in reducing the effects of over 70 diseases that may otherwise have a significant impact on the human body.
GSCG Expands to Cancun, Meets the Demands of US Patients
The Global Stem Cells Group recently announced the opening of a new office in Cancun, Mexico. This decision was driven by the increasing demand for cell therapy procedures by patients in the United States and the lack of facilities able to provide these individuals with professional and quality services. Doctors specializing in regenerative medicine are also having a hard time fulfilling the needs of patients who wish to consider cell therapy as a potential treatment option.
GSCG’s Facilities in Cancun
This is not the only local office that GSCG operates in Cancun, as the company now has two facilities within this location. These offices include a stem cells laboratory and a general medical facility. Over the years, GSCG has become an established name in the stem cell field, providing thousands of doctors the opportunity to offer cell therapy as a treatment option for patients with degenerative diseases. This therapy may assist in the regeneration of damaged and diseased tissues in the patient’s body.
Ensuring Quality and Safety at GSCG
Even though a previous office had established a presence for GSCG in Cancun, the company announced that the opening of the new office means they have now officially created a permanent presence for the brand in the area. GSCG’s new facility in Cancun is equipped with the latest advancements in the field and provides quality services that ensure patients have a trusted medical facility where they can undergo cell therapy as part of a regenerative medicine treatment plan.
Support for US Doctors in Regenerative Medicine
Doctors in the field of regenerative medicine within the United States can now turn to this facility to offer their patients an additional treatment option, apart from the standard pharmaceutical protocols. The stem cell laboratory can assist in analyzing patient data and finding a matching donor for those in need of stem cell treatment. GSCG’s facility can help with the culturing of expanded autonomous cells and other cell types utilized in stem cell therapy. A detailed approach is taken, considering the age of the donor, which provides an overview of the autonomous stem cell age. The patient being treated is also closely considered, as there are different methods of introducing these stem cells to the body for enhanced efficacy.
Advanced Treatment Methods at GSCG
A more aggressive method is often needed for patients with conditions such as autism, Alzheimer’s disease, and kidney-related conditions. Other conditions treated include pulmonary diseases and Parkinson’s disease. In cases of stroke, the vascular arteries, along with the carotid artery, are targeted with the therapy for a more effective approach to assisting with the regeneration of damaged structures in the patient’s brain. This ultimately leads to a significant improvement in the delivery of stem cells to the patient’s body, enhancing the overall efficacy of the treatment.
Comprehensive Patient Care at GSCG
When procedures are provided to the patient through the GSCG, an appropriate ICU unit is made available to ensure the patient is provided with adequate care during the operation and recovery. The utilization of recent technological advancements at GSCG laboratories can also assist in providing a more economical approach to cell therapy. Allogenic stem cell procedures can be addressed using techniques that reduce the costs involved, making these treatments available to patients at a lower price.
Advanced Cultivation Techniques at GSCG
The utilization of autologous stem cell transplantation offers an opportunity for patients to undergo stem cell therapy when a sibling does not provide an ideal match. Cells can be cultivated from the bone marrow and treated with specific drugs to yield targeted results. In the case of malignant cells, bone marrow can be treated with monoclonal antibodies or cytotoxic drugs to target such cells when transplanted back into the patient. Appropriate storage solutions are also considered to assist with the cultivation process and reduce the risk of enzymatic treatment leading to the differentiation of cells into other types. The culture condition becomes crucial for the expansion of stem cells, utilizing mediums and techniques that ensure successful cultivation and expansion.
The GSCG’s Role in the New FDA Warnings
The FDA’s recent warnings and actions against certain stem cell therapy products have caused a limitation in access to these treatments. With the seizure of services provided by companies affected by the FDA’s actions, patients may not be sure where to turn. This issue also affects many doctors in regenerative treatments and medicine, as they need to ensure that the facilities they use for patients offer safe procedures.
GSCG’s Trusted Services
This is where the GSCG comes into the picture. With an established presence in Mexico, doctors now have access to a facility with a history of providing quality stem cell therapy services to patients with qualifying diseases. The company is trusted and has performed stem cell therapy procedures that have led to successful results.
Hope for Patients Through GSCG
These treatments may help repair neurological problems caused by disorders like Parkinson’s disease, stroke, or spinal cord injuries. They may also offer new hope to people with diabetes, heart disease, and those who have suffered cardiovascular damage due to a heart attack.
Conclusion
Even though recent FDA publications have raised concerns regarding stem cell therapy, many patients are still interested in undergoing these treatment procedures. A significant number of studies have provided evidence on the efficacy of treating tissue damage caused by various conditions through stem cell therapy. Patients interested in undergoing stem cell treatment are now advised to turn their interest to Stem Cells Centers in Cancun and related regions, where highly specialized and experienced doctors can provide professional service.
- Published in Corporate News / Blog
ISSCA Set to Welcome 13 Speakers at Upcoming Symposium in Miami
The regenerative medicine symposium will be held on the University of Miami campus on October 24, 2019.
MIAMI LAKES, Florida—The International Society for Stem Cell Application (ISSCA) has announced its lineup of speakers for its upcoming Regenerative Medicine Symposium. The symposium will be held on the University of Miami campus in the Donna E. Shalala Student Center on October 24, 2019 and features an impressive lineup of global authorities in regenerative medicine.
ISSCA is a global leader in stem cells research, applications, and education, partnering with major global institutions and locations worldwide to host its independent medical congresses. This year’s symposium hosted on the University of Miami campus will feature industry experts from seven countries, including Spain, Argentina, Bolivia, Colombia, Ecuador, Mexico, and the US. Some notable speakers include the following:
- Dra. Silvina Pastrana from Argentina, who serves as the Medical Director of the Stem Cells Center Buenos Aires. Dra. Pastrana will discuss her experiences in treating patients who suffer from osteoarthritis conditions.
- Dr. Pedro Sanchez hails from Colombia and is a highly regarded regenerative medicine and orthopedic specialist. He currently practices in Bogota where he offers stem cells treatments for people who suffer from orthopedic conditions.
- Dr. Miguel Guillermo Garbe of Spain serves as the president of the country’s Regenerative Medicine Association. He currently leads a seminal investigation on how to increase cardiological function in patients who have suffered from strokes.
- Dr. Roberto Blum from Ecuador has worked in the stem cells field for over 10 years and will share his industry experience with attendees.
- Dr. Victor Pereyra, an Argentinian neurosurgeon, will lecture on how ozone therapy and stem cells could help patients suffering from spinal injuries.
“The symposium will provide an informative day where physicians can learn from global leaders in the stem cells industry with the hopes of bringing new knowledge and treatment options back to their practices for their patients suffering from degenerative diseases.”
To learn more about the ISSCA and its Miami symposium, visit
http://www.stemcellsgroup.com/.
.
- Published in Press Releases
GSCG Meets Demands of Patients Seeking Stem Cells Treatment with New Facilities
The group’s new permanent office in Cancun will help streamline the patient experience by providing holistic support throughout the treatment process.
MIAMI LAKES, Florida—The Global Stem Cells Group (GSCG) today announced that it will be opening a permanent office in Cancun, Mexico. The decision to expand its corporate presence into Cancun was based on the rising demand for patients seeking stem cells treatments at the group’s facilities already established in Cancun. The GSCG maintains two regenerative medicine operations in Cancun, which include medical facilities and a stem cells laboratory.
GSCG’s Cancun facilities provide state-of-the art options for patients seeking stem cells treatments that are currently not available in the US. While these protocols and products are currently unavailable within the US’s borders due to regulations, the Cancun center has still adopted the FDA’s frameworks for safe and effective regenerative medicine products and therapies. Guided by these protocols, physicians at the center deliver safe and effective stem cells therapies and products that can help patients suffering from degenerative diseases recover more quickly than with traditional treatment protocols.
With the establishment of a permanent office presence in Cancun, the GSCG seeks to provide a seamless holistic experience for patients seeking treatment at its facilities. With its office located near its medical center and laboratory, the GSCG team can more closely monitor the treatment process from the time the patient arrives at the Cancun airport to the time their treatment is complete and they return to the US.
“The Global Stem Cells Group is pleased to establish a permanent presence in Cancun with the opening of this new office location,” said Benito Novas, CEO of the Global Stem Cells Group. “By expanding our presence, we will be able to open up additional treatment opportunities for physicians in the US who may be limited by FDA regulations in ensuring their patients receive the critical stem cells therapies they need. US patients looking for relief from regenerative diseases are welcome at our Cancun center and will appreciate our state-of-the art facilities and top-notch care.”
To learn more about the Global Stem Cells Group and its latest efforts, visit http://www.stemcellsgroup.com/.
.
- Published in Press Releases
- 1
- 2




