How to Boost Your Immune System to Fight Cancer
The human immune system helps to protect the body against illness and infection caused by bacteria, viruses, fungi, and parasites. This process is achieved by a collection of reactions and responses made by the body to damage these infected cells. It is also called immune responses.
This immune system is very crucial in cancer patients to help fight cancer. It identifies cancer as abnormal and destroys it, but this is not enough to rid the body of cancer altogether. In most cases, cancer will weaken this immune system because it spreads into the bone marrow that produces blood cells that fight against infections and cause the bone marrow to decrease its blood cell production.
When the immune system is unable to recognize these cells as abnormal, cellular growth will occur, causing uncontrolled growth and division of cells, leading to cancer.
The Importance of a Strong Immune System in Cancer
Cancer has been a serious and sensitive topic for both patients and doctors. Being diagnosed with cancer is frightening and tormenting, but due to the latest treatment methods, recovering from this disease is increasingly possible. Living with cancer often brings an initial psychological crisis, but surprisingly it affects not only you but also your family and others close to you.
Cancers, especially genetic cancer, can’t be prevented, so it is essential to be proactive about your health. If you’re diagnosed with cancer, some individual lifestyles will help improve your cancer care, which includes managing stress. Reducing your stress level can help you to maintain physical and mental health; you can use relaxation techniques such as meditation and yoga. Getting enough sleep is also crucial for patients living with cancer; this will help improve their health, mood, and coping ability. Also, it is best to limit exposure to toxins that can increase one’s risk of being exposed to other deadly diseases.
Healthy Feeding Habits
A healthy feeding habit is critically important and can help manage cancer side effects, improve health, and quick recovery. Some tips to help you develop a good and healthy diet include:
- Consuming assorted types of vegetables in all your meals. Vegetables are essential and should not just be a side dish but the centerpiece of your meal.
- Consuming foods rich in fiber like grains, beans, peas, nuts, and seeds.
- Consuming less red meat, like pork, lamb, goat, and bison.
- Including probiotic and prebiotic foods to support a healthy gut. Probiotic foods include yogurt or other fermented vegetables, and prebiotic foods include raw or cooked onions, dandelion greens, and legumes.
- Avoiding processed and canned foods.
- Avoiding foods high in calories and low in nutrients like fruit-flavored drinks, sodas, candies, and sweets.
- Cutting down on the excessive intake of alcohol.
Exercise and Cancer Care
Other routines that can help improve cancer care include regular exercise. Regular exercise helps to control fatigue, muscle tension, and anxiety in those with cancer, and it is essential, especially during and after cancer treatment, to keep fit and avoid gaining unnecessary weight.
Cancer Treatment Options
There are many cancer treatment options for different types of cancers. Cancer treatments depend on the type and how advanced it is. Some patients with cancer will only have one treatment option, while others will end up with a combination of more than one treatment technique depending on the type and stage of cancer.
Curing cancer is one of the significant challenges of the 21st century. However, few advances to tame the immune system are getting closer to a future where cancer can be a curable disease.
Advances in Immunotherapy
Although cancer treatment is dependent on the stage and type, we need a wide range of therapies that work on a cellular level, like NK cell and stem cell therapies, wide enough to cover the whole spectrum of cancer. Cancer therapies involving the immune system are believed to be a milestone for cancer treatment advances and the development of multiple immune cells as a therapeutic tool.
Our knowledge of cancer has dramatically improved in recent years. It seems increasingly evident that there has been a surge of new technologies, and these technologies could make a great impact on the way we treat cancer, taking us closer to finally curing this deadly disease.
NK Cells: A Promising Cancer Treatment
What Are NK Cells?
One of these promising cancer treatment advances is the natural killer (NK) cell-based immunotherapy. Natural killer cells have emerged as the newest promising therapeutic approach to cancer. Our understanding of the biological processes that take place in cancer is increasing rapidly, leading to this new type of targeted treatment and therapeutic approach. It is hard to overstate the possible importance of NK cells in the future of cancer.
How NK Cells Work
Modern research has shown that NK cells are a type of treatment that stimulates a person’s immune system to fight cancer, and this may give room for a greater number of chances to beat cancer for good. NK cells are also known for their ability to target cancer stem cells, making these cancer stem cells visible to the immune system.
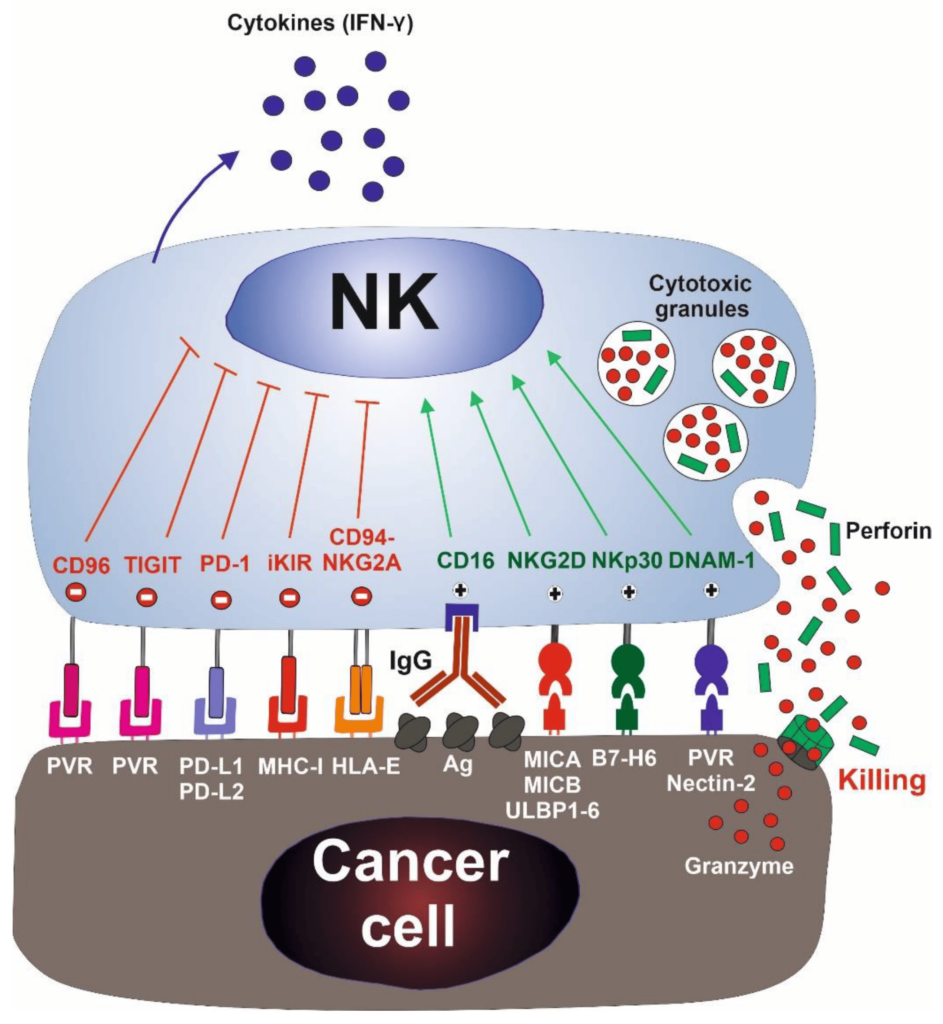
NK cells were first identified in the 1970s as a unique lymphocyte subset that can recognize and kill abnormal cells without prior sensitization to specific tumor antigens, thus preventing the growth of many cancers. During the late 1980s, it was observed that NK cells could destroy a lymphoma cell line that had lost MHC class I surface molecules while the original MHC class I+ cells were resistant to lysis. This led to the formation of the “missing self-hypothesis,” which states that NK cells can sense the absence of “self” MHC class-I molecules on target cells. In recent years, the hypothesis was confirmed by the inhibitory and activating NK cell receptors.
Mechanisms of NK Cell Action
This discovery indicates that autologous cells are not killed by NK cells, thanks to an appropriate expression of all self-HLA alleles, preventing the killing of autologous cells. In contrast, a wide spectrum of cancer types can be killed due to the loss of HLA molecules and the expression/overexpression of ligands for NK cell activating receptors.
NK cells first recognize the tumor cells via stress or danger signals from their sensors. Then, the activated NK cells directly decimate the target tumor cells through at least four mechanisms: cytoplasmic granule release, death receptor-induced apoptosis, effector molecule production, or antibody-dependent cellular cytotoxicity (ADCC).
Furthermore, NK cells act as regulatory cells when they reciprocally interact with dendritic cells (DCs) to improve their antigen uptake and presentation. This action helps in facilitating the generation of antigen-specific cytotoxic T lymphocyte (CTL) responses that are vital in dictating foreign invasion and tumor cells.
By producing cytokines such as IFN-γ, activated NK cells induce CD8+ T cells to become CTLs. Activated NK cells can also help in the differentiation of CD4+ T cells toward a Th1 response and promote CTL differentiation. Cytokines produced by NK cells might even have the unique ability to regulate antitumor cells, making them more proactive.
Enhancing NK Cell Therapy
Accordingly, the cytokine gene transfer approach induces NK cell proliferation, increasing survival capacity, further enhancing their activation, and making them more potent in malignant cell multiplication dictation. NK cell scientists have assured that by using NK cell lines and modifying genes such as IL-2, IL-12, IL-15, and stem cell factor (SCF), they have demonstrated the ability of NK cells to restore their cytotoxic capacity as well as increase their proliferative rate, survival ability, and in vivo antitumor activity. However, the specificity of NK cells is still limited, and studies are still passing through phases. The approach focuses on retargeting NK cells to tumor cells by gene transfer of chimeric tumor-antigen specific receptors.
NK therapy is promising research; it raises the prospect of a “one-end-solution” to many different types of cancers across the globe. If the studies and experiments work out well, NK cells will be able to increase the activity of CD4+, CD19, and other important disease-fighting cells of the body.
The Future of NK Cell Therapy
There is no doubt that this is an unimaginable feat, both in advancing our basic knowledge about regenerative medicine and for the possibility of future cancer treatment advances.
- Published in Corporate News / Blog
Clinical trials on NK cells give hope for many people who are suffering from cancer.
Induced Pluripotent Stem Cells (iPSCs)
Induced pluripotent stem cells (also known as iPS cells or iPSCs) are a type of pluripotent stem cell that can be generated directly from a somatic cell. Pluripotent stem cells hold promise in the field of regenerative medicine. Because they can propagate indefinitely, as well as give rise to every other cell type in the body (such as neurons, heart, pancreatic, and liver cells), they represent a single source of cells that could be used to replace those lost to damage or disease.
Natural Killer Cells and Their Role
Natural killer (NK) cells are a type of cytotoxic lymphocyte critical to the innate immune system. The role NK cells play is analogous to that of cytotoxic T cells in the vertebrate adaptive immune response. NK cells provide rapid responses to virus-infected cells, acting at around three days after infection, and respond to tumor formation.
Typically, immune cells detect the major histocompatibility complex (MHC) presented on infected cell surfaces, triggering cytokine release and causing apoptosis. NK cells are unique, however, as they can recognize stressed cells in the absence of antibodies and MHC, allowing for a much faster immune reaction.
Clinical Trials on NK Cells
Overview of NK Cell Clinical Trials
In a first clinical trial, a natural killer cell immunotherapy derived from induced pluripotent stem cells is being tested for safety in 64 patients with a variety of solid tumors. The first subjects used for the study received the cells in February at the University of California, San Diego (UCSD) Moores Cancer Center and MD Anderson Cancer Center.
Targeted Cancer Types
This study targets late-stage cancer patients with solid tumors, including lymphoma, colorectal cancer, and breast cancer. The FT500 NK cells do not undergo any further alterations after their derivation from induced pluripotent stem cells (iPSCs), offering the possibility of a quicker, ready-made treatment.
Human Embryonic Stem Cells and iPSCs
Human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) provide an accessible, genetically tractable, and homogenous starting cell population to efficiently study human blood cell development. These cell populations provide platforms to develop new cell-based therapies to treat both malignant and nonmalignant hematological diseases.
NK Cells and Cancer Cell Targeting
The NK cells are immune cells in the same family as T and B cells and are very good at targeting cancer cells for destruction. Laboratory experiments have shown they do so by attacking cells that have lost their significant self-recognition signals that tell the immune system not to attack. This is the phenomenon that can happen among cancer cells but not healthy cells. Experts are not sure how many cancer cells lose that signal. Researchers are hopeful that the clinical trial can help determine which cancer patients could benefit the most from NK cell treatment.
iPS Clones and Tumor Formation
The ability to induce pluripotent stem cells from committed, human somatic cells provides tremendous potential for regenerative medicine. However, there is a defined neoplastic potential inherent to such reprogramming that must be understood and may offer a model for critical understanding of events in the formation of tumors. Using genome-wide assays, we identify cancer-related epigenetic abnormalities that arise early during reprogramming and persist in induced pluripotent stem cell (iPS) clones. These include hundreds of abnormal gene silencing events, patterns of aberrant responses to epigenetic-modifying drugs resembling those for cancer cells, and the presence in iPS and partially reprogrammed cells of cancer-specific gene promoter DNA methylation alterations.
Modification of T Cells for Cancer Therapy
Progress in Adoptive T-cell Therapy
Progress in adoptive T-cell therapy for cancer and infectious diseases is hampered by the lack of readily available antigen-specific human T lymphocytes. Pluripotent stem cells could provide an estimable source of T lymphocytes, but the therapeutic potential of human pluripotent stem cell-derived lymphoid cells generated to date remains uncertain.
CAR T-Cell Therapy
Recently, some approved cell therapies for cancer also rely on modifying T cells, in those cases to produce cancer cell–binding chimeric antigen receptors (CARs), and have been effective in treating certain cancers such as leukemia.
Application of CAR T-Cell Therapy in Solid Tumors
The CAR T technology has wowed the field by all but obliterating some patients’ blood cancers, but solid malignancies present new challenges.
Therapies containing such chimeric antigen receptor (CAR) T cells have been approved for some types of so-called liquid cancers of the blood and bone marrow, such as large B-cell lymphoma and B-cell acute lymphoblastic leukemia. However, the approach has not had as much success for solid tumors.
Challenges and Innovations
Serious research into the therapy for brain cancer started almost 20 years ago after cancer biologist Waldemar Debinski, then at Penn State, discovered that the receptor for the immune signaling molecule interleukin 13 (IL-13) was present on glioblastomas, but not on healthy brain tissue. The receptor thus seemed like an excellent target to home in on cancer cells while sparing healthy ones. The CAR spacer domain that spans the immune cells’ membranes and its intracellular co-stimulatory areas, as well as the process used to expand cells outside the body, boosts the T cells’ activity.
CAR T-Cell Therapy: A Safer Approach
While managing CAR T-cell therapy toxicity could help keep already-designed treatments on their march to the clinic, many immunotherapy companies are also working to develop a new generation of inherently safer therapies, yet just as efficient. A crucial part of achieving this goal will be improving CAR specificity for target cells. With current treatments, the destruction of normal cells is often an unavoidable side effect when healthy tissue carries the same antigens as tumors; noncancerous B cells, for example, are usually casualties in CD19-targeted therapies.
Delivery Challenges
CAR T delivery is a challenging factor in the treatment of solid tumors and other unknown forms of tumors. With non-solid cancers, cells are administered by a blood infusion, and once in circulation, the CAR T cells can seek out and destroy the rogue cells. For solid tumors, it’s not so simple.
Future Directions
The main drawback of taking cells from a patient and developing them into a cellular immunotherapy product is that the process can take weeks.
Patel tells The Scientist, “But for the majority of patients who may not be a candidate or may not have time to wait for such an approach, the idea that there’s off-the-shelf immunotherapy that could potentially act as a living drug against their cancer, I think is a fascinating concept.”
- Published in Corporate News / Blog



