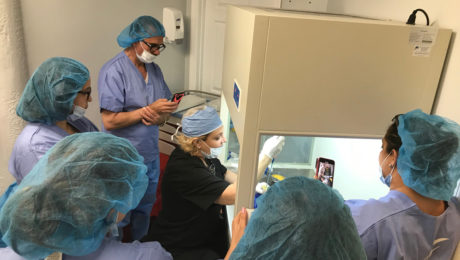
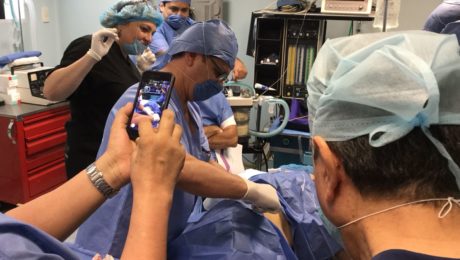
ISSCA to Conduct First Hands On Regenerative Medicine Training after Global Pandemic
he COVID19 pandemic has brought about a ‘new normal’ of social-distancing, closed businesses and self-isolation. After several months of inactivity brought about by this virus and the period of self-isolation, the International Society for Stem Cell Application is ready to begin the process of resuming our schedule of training courses,
Florida (PRUnderground) May 19th, 2020
Now, more than ever, interest in the immune system and its health has breached our collective consciousness, both phycicians and scientist are turning to regenerative medicine as a way to strengthen it. Recent breakthroughs in the field have led to a newly devised program– one with a proprietary protocol for strengthening the immune system, furthering a person’s ability to fight and prevent disease.

This inaugural training will take place in Washington over the course of two days, during which we will be training a group of five doctors in the latest regenerative medicine techniques. We will go over our traditional program providing physicians with all of the tools they will need to begin incorporating regenerative medicine into their own practice
The International Society for Stem Cell Application is an independent, non-profit organization whose objective is to become the gold standard of regenerative medicine. It accomplishes this through hosting conferences and specialized certification courses, publishing and sharing clinical research, and setting protocols in the field of regenerative medicine. Through this partnership, we are seeking to spread knowledge on the current applications of cellular therapies by building a platform for doctors to discuss and debate the newest advancements in the field. These conferences have been held in dozens of countries, and work to create a global network of regenerative medicine researchers and practitioners.
If you’d like to know more information about our program, or the courses that we offer around the world, please check out by visiting our website at: www.issca.us
About International Society for Stem Cells Applications
The International Society for Stem Cells Applications (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology, and the practice of regenerative medicine. Incorporated under the Republic of Korea as a non-profit entity, the ISSCA is focused on promoting excellence and standards in the field of regenerative medicine.
ISSCA bridges the gaps between scientists and practitioners in Regenerative Medicine. Their code of ethics emphasizes principles of morals and ethical conducts.
At ISSCA, their vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training, and certification. ISSCA serves its members through advancements made to the specialty of regenerative medicine. They aim to encourage more physicians to practice regenerative
medicine and make it available to benefit patients both nationally and globally.
For more information, please visit https://www.issca.us/ or send an email to info@stemcellsgroup.com
- Published in Press Releases
GLOBAL STEM CELLS GROUP will host ISSCA Regenerative Medicine Training
After several months of silence due to the mandatory shutdown in place, the training division of Global Stem Cells Group –known as ISSCA Training– has officially announced the reopening of its regularly scheduled training dates Around the world
Miami, FL (PRUnderground) May 20th, 2020
“The future’s looking bright for our Regenerative MedicineTraining program, we’ve taken our course and updated it, reinforcing it with new information and quality protocols that’ll help doctors and their patients manage this ‘new normal’ as well as they can, and we’re very excited to be able to be bringing this new information to physicians around the world– but we’ve decided to start small for now, with the reinstatement of our headquarters’ operations,” — Benito Novas, CEO

Because of the growing interest in the industry , ISSCA Regenerative MedicineTraining has updated its program with new protocols focused on the optimization of the body at the cellular level. Notably, their upcoming June course will also include live practice patients in the treatments of autologous cell therapy, which includes PRP, adipose-derived stem cells, bone-marrow derived stem cells, exosomes, Umbilical Cord Blood Cells, and other novel techniques.
Stem Cell Training is a company that seeks to instruct medical professionals around the world on regenerative medicine techniques. They do this by sharing breakthrough scientific research on stem cell therapy and treatment, as well as developing and offering courses, seminars, and workshops based on this research.
About the Global Stem Cells Group:
Global Stem Cells Group is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2010, the company combines dedicated researchers, physicians ,patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge Regenerative Medicine treatments.
With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Groups corporate mission is to make the promise of Regenerative medicine a reality for patients around the world. With each of GSCGs six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art regenerative technology
- Published in Press Releases
Global Stem Cells Group Announces Launch of Vita Novas In-Home IV Infusion Service
Its no secret that a quality IV infusion can boost health and energy. Vita Novas makes this more simple than ever performing IV services than revitalize the body and help it regenerate, right at home.
Miami , Florida (PRUnderground) May 20th, 2020
The interest in boosting the immune system in a safe and effective way is a topic on more minds than ever. In exciting news, Global Stems Group has announced the launch of a new company, Vita Novas who make this both simple and dependable. Vita Novas offers an IV infusion packages are a mix of stem cells, essential fluids, electrolytes, vitamins and antioxidants as a preventive solution for having an optimal immune system. This IV infusion is delivered at home, in an office, or at a hotel, by certified healthcare professionals. With the global pandemic shutting doors of physicians for elective and health-enhancing non-vital procedures going to get IV treatments in most communities is difficult, if not impossible. Vita Novas is opening the doors for these life-enhancing treatments to be accesses in an easy and convenient way.
“The demand for preventive health services using IV infusion is greater than ever,” commented Benito Novas, CEO of the company. “What we are doing, by offering these at home and offices, is not just making things more convenient but also following social distancing requirements. Its truly making the right thing available at the right time and place.”
Vita Novas provides a teleconsultation with a Doctor to determine what meets a patient’s needs best. Three different vitamin and nutrient vitamin infusion packages are available.
The Lighting Package consists of two sessions of IV administration of vitamins (Vitamin C, B-Complex vitamins, and trace minerals), with a patient follow up recommending dietary changes and oral supplements to maintain good health.
The Shield Package is three sessions of IV administered (high dose Vitamin C, Methylcobalamin, Zinc sulphate, Glutathione and five billion flow exosomes). This has been shown have a powerful effect in regulating regenerative processes in the body and increase cell to cell communication. This has been used to treat COVID-19 patients with exciting results, something clearly at the front of people’s minds of all ages and lifestyles.
The Fighter Packages is the platinum offer from Vita Novas. This combines IV administration of high dose micronutrients (amino acids, glutathione), vitamins (B-Complex, methylcobalamin, Folic Acid, Vitamin C), and trace minerals (zinc, copper, magnesium) along with 30 million live stem cells in 5 sessions. This can modulate immunity and assist in the regeneration process of damaged or infected tissue in a very dramatic way.
Vita Novas is currently positioned to be able to launch the program in ten different countries with the number rising to over 25 within six months. The near and far future are bright for the company and its potentially life-changing services.
About the Global Stem Cells Group:
Global Stem Cells Group is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2010, the company combines dedicated researchers, physicians ,patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge Regenerative Medicine treatments.
With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Groups corporate mission is to make the promise of Regenerative medicine a reality for patients around the world. With each of GSCGs six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art regenerative technology
- Published in Press Releases
Bogota Course Will Share the Latest in Stem Cell Treatment Protocols
The two-day certification program is sponsored by ISSCA, will feature three topics new to the group’s Colombian training circuit
MIAMI LAKES, Florida— The International Society for Stem Cell Application (ISSCA), a global leader in stem cells research, applications, and education, has announced their latest certification program to be held in Bogota, Colombia on March 13-14, 2020.
The Stem Cells Certification course will feature a number of educational and hands-on sessions designed to give physicians the tools needed to integrate regenerative treatments into their practices. Registration for the two-day course is limited to eight participants to allow for intensive training and interaction.
During the two-day training, ISSCA faculty will share the latest stem cell protocols, including how to harvest and process stem cells derived from Stromal Vascular Faction (SVF) tissue and bone marrow.
ISSCA faculty will additionally introduce three new protocols never before covered in past courses held in Colombia. The new curriculum includes clinical applications of allogeneic stem cells; using GCell technology, a device that can process SVF without enzymatic compounds; and an introduction to NK cell protocols, the newest regenerative medicine treatment to fight cancer.
“Our two-day training in Bogota will provide physicians with an invaluable opportunity to receive intensive hands-on training and education in some of today’s most effective and cutting-edge stem cells protocols,” said Benito Novas, ISSCA VP of Public Relations. “Physicians will learn from global leaders in the stem cells industry and be able to take practical application strategies back with them to integrate into their own practices to help patients suffering from degenerative diseases.”To learn more about the ISSCA, visit www.issca.us. More information about the Bogota certification course can be found at http://cursocelulasmadre.com/entrenamiento-bogota-programa/
- Published in Press Releases
Mexico City Medical Congress to Showcase the Global Stem Cells Group’s Latest Innovations
The group is sponsoring the event, which will attract physicians from across the globe with an interest in longevity and anti-aging medicine
MIAMI LAKES, Florida— The Global Stem Cells Group (GSCG) is set to sponsor the XI Congreso Mundial de Medicina Antienvejecimiento y Longevidad (World Conference of Anti-Aging and Longevity Medicine) to be held in Mexico City, Mexico on February 16-18, 2020.
The medical congress is expected to attract over 450 physicians and researchers from across the world interested in anti-aging and longevity practices and medical innovations. Over 30 speakers are slated to share information with attendees on a wide range of topics on how to lead a long, healthy life and improve longevity.
The GSCG is set to share a number of its latest innovations with congress attendees, including its newly released GCell technology device. This cutting-edge tool utilizes micrograft technology to harness the natural and powerful restorative capabilities of adipose tissues. Because it is FDA compliant, the device allows physicians across the globe to continue practicing adult stem cells-based procedures.
Additional benefits of GCell technology include shorter treatment times, delivering in-office treatments in around 30 minutes with local anesthesia, as well as less fat collection compared to existing treatments (15 mL versus 50 mL). GCell technology holds exciting implications across a range of medical specialties, including orthopedics, dermatology, cosmetic gynecology, aesthetics, and hair loss.
In addition to its GCell technology, the GSCG will also feature its newest line of stem cells products derived from first-tissue exosomes. Cellgenic Flow Exosomes utilizes the latest science and research available in cellular therapies to deliver a non-surgical approach to creating regenerative responses in a broad range of treatments. The product utilizes exosomes, which replicate the signals given out by stem cells, versus actual stem cells. Exosomes play a pivotal role in cell-to-cell communication and are involved in a wide range of physiological processes. These particles transfer critical bioactive molecules such as proteins, mRNA, and miRNA between cells and regulate gene expression in recipient cells.
“The XI Congreso Mundial de Medicina Antienvejecimiento y Longevidad is one of the world’s premier events connecting physicians and researchers with today’s most innovative treatments and technologies utilizing regenerative medicine,” said Benito Novas, CEO of the GSCG. “As a worldwide leader in training, education, and innovative products in the field of regenerative medicine, the GSCG is pleased to sponsor this congress and share its exciting new portfolio of products with physicians from across the world.”
To learn more about the Global Stem Cells Group and all of the group’s latest news and innovations, visit http://www.stemcellsgroup.com/
- Published in Press Releases
Two Global Stem Cells Group Officials to Speak at Paraguay Conference
The group is set to sponsor the 2nd Congress of Aesthetic Medicine and Regenerative Gynecology to be held in Paraguay
MIAMI LAKES, Florida—The Global Stem Cells Group (GSCG) has announced its sponsorship of the 2nd Congress of Aesthetic Medicine and Regenerative Gynecology to be held on March 12-13, 2020 in Asuncion, Paraguay. The GSCG’s CEO Benito Novas and Development and Research Director Dra. Maritza Novas will each deliver key lectures during the annual medical congress, which will focus on advancements in the fields of aesthetic medicine and regenerative gynecology.
Benito Novas serves as the GSCG’s CEO and will lead a lecture on regenerative medicine marketing strategies. His talk will give practical marketing tools and advice to physicians running their own private practices in aesthetic medicine. In addition to his role as CEO with the GSCG, Novas also serves as the Head of Public Relations for the International Society for Stem Cell Application (ISSCA) and has authored two books on marketing strategies for clinics in the regenerative medicine industry, Your Aesthetic Practice: What Your Patients Are Saying and Marketing Digital en Su Clínica Estética (https://www.thriftbooks.com/a/benito-novas/2752773/).
Dra. Maritza Novas serves as the GSCG’s Development and Research Director as well as the US Director for the ISSCA. She will deliver the opening lecture for the congress, discussing clinical applications of regenerative medicine in the aesthetic field. Her lecture will be informed by clinical case studies demonstrating the efficacy of regenerative treatments in aesthetics. Dra. Novas is well-renowned in the field of regenerative medicine for making a profound impact on her patients through innovative treatment protocols. She has additionally trained countless doctors looking to add regenerative medicine to their practices.
On March 14, following the conclusion of the congress, representatives from the GSCG will also offer a certification course in cellular therapy. The course is designed to help all physicians who want to add stem cells therapies based on birth tissue derived components to their practices. Attendees will learn about the latest stem cell products, such as exosomes, cord blood, and amniotic fluid. Additional topics to be covered will include how to choose the right products to treat specific conditions and details about how to handle the manufacturing process, from selecting a healthy donor through quality control and storage at the medical office.
“The Global Stem Cells Group is pleased to serve as a sponsor for the 2nd Congress of Aesthetic Medicine and Regenerative Gynecology,” said Benito Novas. “We look forward to sharing today’s best research on marketing strategies to help regenerative medicine practices grow their success and influence as well as sharing clinical applications of stem cells treatments in aesthetic medicine. As a global leader in stem cells education, we always look forward to these events to help more physicians gain the knowledge and skills necessary to bring life-saving treatments to more patients across the globe.”
To learn more about the Global Stem Cells Group and all of the group’s latest news and innovations, visit http://www.stemcellsgroup.com/.
- Published in Press Releases
Global Stem Cells Group to Sponsor Miami Congress on Anti-Aging and Longevity
The group will launch its innovative GCell technology and a new online course at the event, while CEO Benito Novas will lead a lecture about marketing in the regenerative medicine field
MIAMI LAKES, Florida—The Global Stem Cells Group (GSCG) is set to sponsor the Second Intercontinental Medical Congress on Anti-Aging and Longevity (https://miami2020.semal.org/) hosted by the Spanish Society of Anti-Aging Medicine (SEMAL) and the Federation of Latin American of Anti-Aging Medicine (FISMAL). The congress will be held on February 6-9, 2020 in Miami, Florida at the Hampton Inn & Suites by Hilton Miami Brickell.
During the event, the Global Medical Group, a subsidiary of GSCG, will launch its GCell micrograft technology, an autologous tissue suspension that boasts minimal manipulation. The product has application implications for a wide range of medical uses, including orthopedics, dermatology, cosmetic gynecology, aesthetics, and hair loss.
GCell technology will allow physicians utilizing regenerative medicine to continue practicing adult stem cells-based procedures thanks to the product’s adherence to FDA compliance standards since the procedure does not utilize enzymes.
GCell technology is a closed-system medical device that harnesses the natural and powerful restorative capabilities of adipose tissue. The GCell SVF (Stromal Vascular Fraction) procedure can be performed in office in around 30 minutes, while existing procedures can take up to two hours. It also utilizes only local anesthesia, and performing physicians only have to collect 15 ml of fat instead of the 50 ml required for traditional protocols.
During the congress, the GSCG will also be launching its new online stem cells course, which will benefit doctors interested in learning about the newest stem cells protocols and technologies. The course will specifically focus on stem cells protocols based on first tissue derived compounds, such as exosomes, cord blood, and amniotic fluid.
In addition to launching GCell technology and its new course, the GSCG’s CEO and founder Benito Novas will be giving a lecture in the main room. He will be speaking to attendees about the newest challenges facing the regenerative medicine filed and what types of marketing strategies practitioners should implement to run a successful practice integrating regenerative medicine.
“The Global Stem Cells Group is looking forward to attending the Second Intercontinental Medical Congress on Anti-Aging and Longevity to talk with attending physicians and researchers about the technological and educational advances we are making to help move the field of regenerative medicine forward,” said Novas.
To learn more about the Global Stem Cells Group and all of the group’s latest news and innovations, visit http://www.stemcellsgroup.com/.
- Published in Press Releases
Global Stem Cells Group Enters Training Agreement with Moroccan Hospital
The agreement between Global and CHU Mohammed VI in Marrakech will help enhance staff preparation and clinical offerings in regenerative medicine
MIAMI LAKES, Florida— Stem Cells Training Inc., a subsidiary of the Global Stem Cells Group (GSCG), has announced that it has signed an onsite training agreement with Centre Hospitalo-Universitaire Mohammed VI Marrakech (CHU). GSCG faculty will travel to Marrakech, Morocco to provide this invaluable two-day training to CHU’s staff on February 14 and 15, 2020.
The agreement names the GSCG as CHU’s provider of training for the facility’s regenerative medicine staff. Through the deal, the GSCG will provide preparation and education in the latest regenerative medicine techniques and stem cells protocols, focusing on those utilizing adult stem cells and birth tissue derived from stem cells. In addition to on-site training, the agreement also names the GSCG as CHU’s provider for all necessary equipment to implement regenerative medicine treatments.
The additional training and supplies offered through the agreement will allow CHU to incorporate the industry’s most effective stem cells protocols into the hospital’s treatment options.
The Centre Hospitalo-Universitaire Mohammed VI is a regional leader in quality patient care, student education, physician training, and innovative medical research. Located in Marrakech, CHU provides the bustling city with short-, medium-, and long-range care solutions. Counted among its innovative institutional projects, CHU’s Culture and Health program is committed to making the hospital a more human environment for patients seeking care.
“The agreement between the Global Stem Cells Group and the Centre Hospitalo-Universitaire Mohammed VI Marrakech is an important one for both partners,” said Benito Novas, CEO of the Global Stem Cells Group. “As a university institution, CHU is interested in leading clinical trials to prove the safety and efficacy of regenerative medicine treatment protocols—something the GSCG has a vested interest in. This agreement will allow us to train hospital staff to offer cutting-edge treatments to patients in Morocco while helping stem cells treatments gain more traction globally, making them more viable options for physicians to incorporate into their practices.”
With the newly signed agreement, the GSCG continues to advance its mission to expand its presence across the global, especially focusing on North America and the Middle East. Physicians looking to grow their practices by offering regenerative medicine treatments can benefit from the GSCG’s trainings, which are conveniently held onsite at physicians’ home facilities, no matter where they are located globally.
To learn more about the onsite training opportunities offered by the Global Stem Cells Group, visit https://www.stemcelltraining.net/onsitetraining/. To learn more about the Centre Hospitalo-Universitaire Mohammed VI Marrakech, visit www.chumarrakech.ma.
- Published in Press Releases
ISSCA Announces its 2020 Schedule of Regenerative Medicine Congresses
The group will host six events across the globe, combining networking and education for practitioners of regenerative medicine
MIAMI LAKES, Florida— The International Society for Stem Cell Application (ISSCA), a worldwide leader in regenerative medicine education, has announced its slate of 2020 medical congresses. The group’s calendar includes dates at six locations across the globe
Through its congresses, the ISSCA seeks to create a platform through which physicians can collaborate, share data, initiate discussions, and exchange information that may be directly translated to therapeutic applications. All doctors who want to share their medical research are welcome to attend, and the ISSCA welcomes abstract submissions and reviews them on a continuing basis.
For the second year straight, the main topic discussed at ISSCA’s congresses will be how cellular products derived from birth tissue (allogeneic compounds) have revolutionized the industry by delivering safer, shorter stem cells procedures for practitioners. During each event, lecturers will respond to concerns attendees have about how regenerative medicine advances will satisfy FDA regulations and how new technologies could continue to help patients under new FDA laws.
This year, the ISSCA welcomes three new cities to its agenda—Salvador de Bahia, Brazil; Punta del Este, Uruguay; and Jakarta, Indonesia. The six congresses are as follows:
- May 2020: Mexico City, Mexico
- June 2020: Caracas, Venezuela
- August 2020: Salvador de Bahia, Brazil
- October 2020: Buenos Aires, Argentina
- November 2020: Jakarta, Indonesia
- December 2020: Punta del Este, Uruguay
“We at the ISSCA are looking forward to this year’s calendar of congresses, which will provide invaluable networking and educational opportunities to physicians looking to expand their knowledge on current innovations and best practices concerning regenerative medicine,” noted Benito Novas, ISSCA VP of Public Relations. “Our global congresses expand on ISSCA’s mission to continue to serve as the premier educational resource for physicians across the globe looking to introduce stem cells treatments into their practices.”
To learn more about the ISSCA or to register for an upcoming congress, visit www.issca.us.
- Published in Press Releases
Adimarket Named as Distributor for Innovative GCell Micrograft Technology
Adimarket Named as Distributor for Innovative GCell Micrograft Technology
The partnership between Adimarket and Global Medical Group delivers an FDA-compliant stem cells procedure to physicians practicing regenerative medicine across the globe
MIAMI LAKES, Florida—Adimarket, LLC, a subsidiary of the Global Stem Cells Group (GSCG), has entered an agreement with Global Medical Group (GMG) to distribute the brand’s newest product featuring GCell technology. It will be available for purchase to regenerative medicine practitioners via Adimarket’s online store.
Global Medical Group recently announced the launch of their GCell micrograft technology, an autologous tissue suspension that boasts minimal manipulation. The product has application implications for a wide range of medical uses, including orthopedics, dermatology, cosmetic gynecology, aesthetics, and hair loss.
GCell technology would allow regenerative medicine practitioners to continue practicing their adult stem cells-based procedures, as the product meets FDA compliance standards. GMG’s innovative product uses micrograft technology and is a closed system medical device that harnesses the natural and powerful restorative capabilities of adipose tissue. Because the procedure can be performed without using enzymes, GCell technology maintains FDA compliance.
The GCell SVF (Stromal Vascular Fraction) procedure can be performed in-office in around 30 minutes, while previous procedures could take up to two hours. It also utilizes only local anesthesia, and performing physicians only have to collect 15 ml of fat instead of the 50 ml required for traditional protocols.
GCell technology may hold exciting applications for clients looking for treatment options for wound care, hair loss, orthopedic conditions, or aesthetic concerns.
“We are pleased to partner with the Global Medical Group to offer their cutting edge GCell technology in Adimarket’s product portfolio,” said Benito Novas, CEO of the Global Stem Cells Group. “There is a possibility that this innovative technology could lead to incredible advances in various treatment protocols, including those related to wound care, hair loss, orthopedic injuries, or aesthetics. And because it is FDA compliant, GCell technology will be a highly attractive option to regenerative medicine practitioners in the US and abroad.”
The first unit will be launched in the US market next month. Physicians looking to incorporate GCell technology into their practices will be able to find it at www.adimarket.net.
- Published in Press Releases