Essential Steps for Regenerative Medicine Practitioners
Comprehensive Stem Cell Training
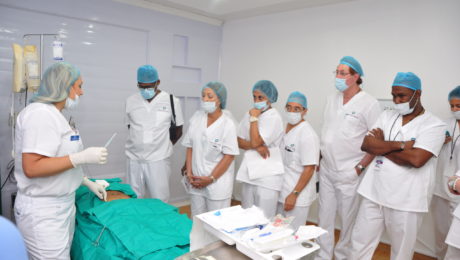
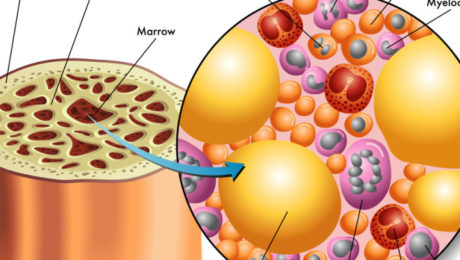
Global Stem Cells Group (GSCG) offers an intensive two-day stem cell training course designed to equip practitioners with the skills to implement cutting-edge regenerative therapies in their clinics. The training includes hands-on experience in techniques such as precision mini lipo-aspiration for fat tissue collection and bone marrow harvesting via the iliac crest.
Didactic Lectures and Practical Application
Participants receive didactic lectures covering stem cell structure, function, and treatment protocols. Practical sessions involve the isolation and harvesting of stem cells from both fat tissue and bone marrow, as well as the extraction of platelet-rich plasma from peripheral blood. Live demonstrations on actual patients ensure thorough understanding and proficiency in performing these procedures.
Certification and Support
Upon completion, GSCG provides written protocols, consent forms, and necessary documentation for seamless integration of stem cell therapies into clinical practice. The course is fully accredited, offering 16 continuing education credits across multiple categories from three universities. Participants also have the opportunity to engage in Institutional Review Board (IRB) clinical studies to further their expertise.
FDA-Approved Products and Partnerships
GSCG collaborates with FDA 510K-approved partners like Emcyte to provide reliable bone marrow and platelet-rich plasma kits. These systems ensure precise processing of bone marrow aspirates, yielding concentrated growth factors and platelets suitable for therapeutic use.
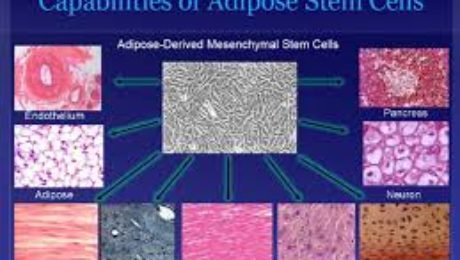
Adipose-Derived Stem Cell Kits
Fat tissue serves as a rich source of mesenchymal stem cells, offering significantly higher yields compared to bone marrow. GSCG’s adipose-derived stem cell kits support the treatment of degenerative diseases and tissue replenishment post-injury. These multipotential progenitor cells are angiogenic and vasculogenic, making them effective in conditions involving ischemia and vascular regeneration.
Laboratory and Equipment Support
GSCG operates a state-of-the-art laboratory in Santiago, Chile, producing high-quality reagents and isolation kits under stringent good manufacturing practices (GMP). Adimarket.net offers a range of products including centrifuges and medical devices essential for implementing stem cell therapies in clinical settings. Training covers the selection and use of equipment required for various therapeutic techniques.
- Published in Corporate News / Blog
Could stem cells repair the damaged brain in Alzheimer’s?
Stem Cell Therapies: A Potential Cure for Alzheimer’s?
Understanding Alzheimer’s Disease

Alzheimer’s disease is a progressive and irreversible brain disorder characterized by symptoms such as disorientation regarding time and place, changes in mood, personality and behavior, memory loss, difficulty solving problems or planning, and difficulty writing or performing other routine tasks. This condition primarily affects people aged 70 years and above, with a higher prevalence in women. It is the main risk factor for dementia among the elderly.
Limitations of Conventional Treatments
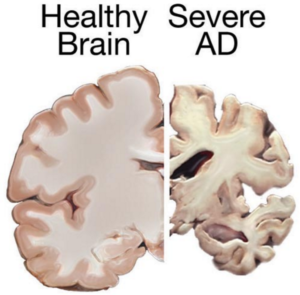
Currently, there is no known cure for Alzheimer’s. Conventional treatments, including both drug-based and non-drug strategies, may help with cognitive and behavioral symptoms but have little to no effect on the disease’s long-term progression. Medications available today cannot stop Alzheimer’s from progressing; they can only temporarily lessen symptoms like confusion and memory loss.
Exploring Stem Cell Therapies
Due to the limitations of conventional treatments, scientists are exploring the possibilities of stem cell therapies in Alzheimer’s treatments. Stem cells have the potential to develop new neurons, replace dead and damaged cells, and deliver neurotrophins to support neuron growth and survival.
Challenges in Developing Stem Cell Therapies
- Transplanting Neural Stem Cells:
- Theoretically, transplanting neural stem cells into the patient’s brain could generate healthy new neurons. However, it remains unclear whether the brain would integrate the transplanted cells effectively and if they could travel to the damaged areas.
- Producing the different types of neurons needed to replace the damaged cells and stimulating the renewal of lost connections between neural cells pose significant challenges.
- Delivering Neurotrophins:
- Neurotrophin proteins support neuron growth and survival, but Alzheimer’s patients produce insufficient amounts. Neural stem cells can produce these proteins, and studies in mice have shown some improvements in memory when treated with stem cells.
- Mesenchymal Stem Cells:
- Mesenchymal stem cells may exert anti-inflammatory effects and help ameliorate Alzheimer’s symptoms. However, there is currently no study proving their safety or effectiveness for this condition.
Research and Studies
Despite the high failure rate of clinical trials and studies on Alzheimer’s treatment, stem cells may still be valuable for studying the behavior of brain cells damaged by the condition. They can also be used for testing various therapeutic approaches and predicting which treatments might help Alzheimer’s patients.
- Induced Pluripotent Stem Cells (iPSC):
- Researchers from the Harvard Stem Cells Institute reprogrammed skin cells from Alzheimer’s patients to create iPSCs. These cells, grown in lab conditions, released the same proteins that form plaques in Alzheimer’s patients. This advancement allows scientists to study Alzheimer’s-affected cells and test new remedies.
- Neuronal Cell Conversion:
- Asian scientists turned human fibroblasts into neuronal cells using a chemical cocktail of small molecules. These findings provide an alternative strategy for modeling neurodegenerative disorders and may play a crucial role in identifying new stem cell-based treatments.
Conclusion
Stem cell therapies hold promise for developing new treatments for Alzheimer’s disease. While many challenges and uncertainties remain, ongoing research offers hope for understanding and potentially curing this debilitating condition.
References
- Published in Corporate News / Blog
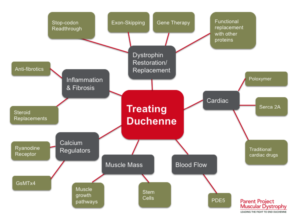
New Stem Cell Research Shows Promising Results for Muscular Dystrophy
Understanding Muscular Dystrophy
Muscular dystrophy (MD) encompasses a group of genetic disorders that cause progressive muscle weakness and loss of muscle mass. These disorders, affecting primarily skeletal muscles, can also impact respiratory and swallowing muscles, leading to severe disability over time.
Types and Impact of Muscular Dystrophy
There are various types of muscular dystrophy, with Duchenne muscular dystrophy (DMD) being the most severe and common form, primarily affecting young boys. DMD is characterized by a genetic mutation that prevents the production of dystrophin, a crucial protein for muscle function. This deficiency leads to progressive muscle degeneration, loss of mobility, and, in many cases, premature death.
Current Treatments and Limitations
Currently, there is no cure for DMD. Treatment focuses on managing symptoms and slowing disease progression through therapies like physiotherapy and steroids. However, these treatments have limitations and often come with significant side effects.
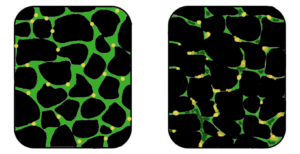
Potential of Stem Cell Therapies for Muscular Dystrophy
Regeneration with Stem Cells
Stem cell research offers promising avenues for treating muscular dystrophy by targeting muscle regeneration. Studies have shown that stem cells isolated from muscle tissue, bone marrow, and blood vessels can potentially regenerate dystrophin-deficient muscle fibers in animal models.
Experimental Success with Stem Cells
Recent studies have demonstrated encouraging results in animal models of DMD. Researchers have successfully restored mobility in dogs and improved muscle function in mice by transplanting stem cells. These studies suggest that stem cells could potentially replace damaged muscle tissue and restore muscle function.
Combining Stem Cell and Gene Therapies
Emerging research also explores combining stem cell therapies with genetic correction techniques. This approach aims to correct the genetic defect responsible for DMD and stimulate muscle regeneration simultaneously, showing promising outcomes in preclinical studies.
Future Directions and Research
While the application of stem cell therapies for DMD in humans is still in its early stages, ongoing research holds significant promise. Future studies will focus on optimizing stem cell treatments, enhancing their safety and efficacy, and ultimately translating these findings into viable therapies for patients with muscular dystrophy.
Conclusion
Stem cell research represents a beacon of hope for advancing treatment options for muscular dystrophy, particularly DMD. As research progresses and clinical trials continue, the potential of stem cells to regenerate muscle tissue and improve quality of life for patients with muscular dystrophy becomes increasingly promising.
References:
- http://www.mayoclinic.org/diseases-conditions/muscular-dystrophy/basics/definition/con-20021240
- http://www.eurostemcell.org/factsheet/muscular-dystrophy-how-could-stem-cells-help
- https://www.mda.org/disease/duchenne-muscular-dystrophy/research
- http://quest.mda.org/article/scientists-bullish-stem-cells-muscle-repair
- http://hsci.harvard.edu/stem-cells-used-treat-muscular-dystrophy-mice
- https://med.stanford.edu/news/all-news/2014/12/stem-cells-faulty-in-duchenne-muscular-dystrophy.html
- Published in Corporate News / Blog
Stem cells may be used for healing damaged lungs
Healing Damaged Lungs with Stem Cells
A new study from the Weizmann Institute of Science reveals promising results using stem cells to repair damaged lung tissue. This breakthrough offers hope for treating conditions like bronchitis, asthma, cystic fibrosis, and emphysema, which collectively affect millions globally.
Bone Marrow Stem Cells for Lung Tissue Regeneration
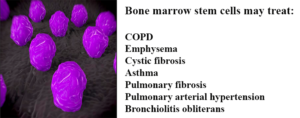
The study focuses on similarities between lung and bone marrow stem cells. Researchers at the Weizmann Institute, led by Professor Yair Reisner, successfully transplanted bone marrow stem cells into mice with lung damage. These stem cells migrated to damaged areas, differentiated into lung tissue, and continued to generate new cells up to 16 weeks post-transplantation.
Future Directions: Lung-Specific Induced Pluripotent Stem Cells (iPSCs)
Advantages of iPSCs Over Bone Marrow Stem Cells
In related research, scientists at Boston University Medical Center generated lung-disease specific induced pluripotent stem cells (iPSCs) from patients with conditions like emphysema and cystic fibrosis. iPSCs offer advantages such as easier cultivation and genetic compatibility, minimizing the risk of rejection in transplants.
Potential for Transplantation and Lung Tissue Differentiation
Studies show that iPSCs can differentiate into endoderm cells, precursors to lung tissue, offering a promising avenue for future treatments of lung diseases.
Conclusion
The research underscores the potential of stem cells, both from bone marrow and induced pluripotent sources, to revolutionize the treatment of lung diseases. Ongoing studies aim to refine these techniques and explore the creation of a bank of lung-specific stem cells for clinical use.
References
- Chava Rosen, Elias Shezen, Anna Aronovich, Yael Zlotnikov Klionsky et al. – Preconditioning allows engraftment of mouse and human embryonic lung cells, enabling lung repair in mice, Nature Medicine, 2015, Link.
- Aba Somers, Jyh-Chang Jean, Cesar A. Sommer, Amel Omari et al. – Generation of transgene-free lung disease-specific human induced pluripotent stem cells using a single excisable lentiviral stem cell cassette, Stem Cells, 2010, 28 (10):1728, Link.
This structure ensures each section is clearly delineated for SEO purposes, incorporating keywords like “stem cells,” “lung tissue regeneration,” “induced pluripotent stem cells,” and specific study references.
- Published in Corporate News / Blog
Adult tissues that serve as sources for stem cells
Where Do Adult Stem Cells Come From?
Adult stem cells are garnering significant interest in the scientific community due to their ability to self-renew and differentiate into various types of cells and tissues. Unlike embryonic stem cells, which can differentiate into multiple cell types, adult stem cells typically generate tissues specific to their origin. This focus on adult stem cells has intensified amidst ethical concerns surrounding embryonic stem cell research, making them pivotal in the study of degenerative conditions such as osteoarthritis, muscular dystrophy, and Alzheimer’s disease.
Sources of Adult Stem Cells
Adult stem cells are found in several tissues, each offering potential therapeutic applications:
Neural Stem Cells (NSCs)
Neural stem cells are multipotent cells that can generate the central nervous system. Recent studies, including research by Japanese scientists, demonstrate their potential to replace dying neurons in conditions like multiple sclerosis and Parkinson’s disease [1].
Hematopoietic Stem Cells (HSCs)
Hematopoietic stem cells, derived from blood or bone marrow, can differentiate into various specialized cells such as white blood cells and red blood cells. They play a crucial role in immune response and blood clotting.
Skeletal Stem Cells (STCs)
Found in bone marrow, skeletal stem cells give rise to bone cells (osteoblasts), cartilage, and hematopoietic stroma, supporting bone structure and function.
Intestinal Stem Cells (ISCs)
ISCs, located in the lining of the intestines, continuously divide throughout life. They are implicated in intestinal cancer development but also offer regenerative potential for treating gastrointestinal disorders.
Liver Stem Cells
In the liver, hepatocytes exhibit stem cell-like behavior during regeneration, replacing damaged tissue. Researchers, such as Dr. Lola Reid, have identified biliary tree stem cells as potential pancreatic precursors, offering insights into diabetes treatment [2].
Conclusion
Research into adult stem cells has expanded our understanding of their therapeutic potential in regenerative medicine. Their diverse sources—from neural tissues to the liver—provide avenues for developing treatments without the ethical concerns associated with embryonic stem cells. With lower rejection rates and higher differentiation capabilities, adult stem cells offer hope for effectively treating degenerative diseases in the future.
References
- MacKlis, Jeffrey D.; Magavi, Sanjay S.; Leavitt, Blair R. (2000). “Induction of neurogenesis in the neocortex of adult mice”. Nature 405 (6789): 951–5.
- Biliary Tree Stem Cells, Precursors to Pancreatic Committed Progenitors: Evidence for Possible Life-long Pancreatic Organogenesis – Link.
This structure enhances readability and SEO by incorporating targeted keywords like “adult stem cells,” “neural stem cells,” “hematopoietic stem cells,” and specific study references.
- Published in Corporate News / Blog
We may soon be able to heal autoimmune disorders with Stem Cells
Autoimmune disorders are conditions where the body’s immune system mistakenly attacks healthy cells, causing damage to tissues and organs. With over 80 known types, including diabetes type 1, lupus, rheumatoid arthritis, celiac disease, and multiple sclerosis, these disorders affect millions worldwide.
Understanding Autoimmune Disorders
The exact causes of autoimmune disorders remain unclear, though triggers like viruses, bacteria, or certain medications may disrupt immune function, leading the body to attack its own tissues. Common targets include red blood cells, skin, connective tissues, blood vessels, and various glands.
Current Treatments and Limitations
Standard treatments for autoimmune disorders primarily involve immune-suppressing medications, which offer temporary relief but do not provide a cure. Researchers are therefore exploring alternative approaches, such as stem cell therapy, to address these conditions effectively.
The Use of Stem Cells in Autoimmune Disorders
Mesenchymal Stem Cells (MSCs) in Immunomodulation
Research indicates that mesenchymal stem cells, derived from sources like bone marrow, exert immunological functions that help maintain immune balance. Studies, including one published in Arthritis Research and Therapy, highlight MSCs’ ability to regulate immune responses and potentially prevent tissue destruction [1].
Hematopoietic Stem Cells (HSCs) for Severe Autoimmune Diseases
Studies, such as those conducted at Harvard Medical School, explore the use of hematopoietic stem cells in treating severe autoimmune diseases like rheumatoid arthritis and multiple sclerosis. Transplantation of HSCs following immunosuppressive therapies has shown promise in achieving disease remission [2].
Neural Stem Cells (NSCs) in Multiple Sclerosis
In multiple sclerosis research, neural stem cells derived from the central nervous system demonstrate neuroprotective and immunomodulatory effects. Studies suggest these cells could potentially repair damaged nerve tissues and improve symptoms in MS patients [3].
Mesenchymal Stem Cells (MSCs) in Rheumatoid Arthritis
Research on rheumatoid arthritis indicates that mesenchymal cells derived from sources like the placenta possess immunosuppressive properties. Studies, such as those investigating human amnion mesenchymal cells, show promise in alleviating arthritis symptoms and reducing disease severity [4].
Challenges and Future Directions
Despite promising results, challenges remain in optimizing stem cell therapies for autoimmune disorders. Researchers must establish precise protocols and address issues like immune rejection and treatment efficacy in larger clinical settings.
References
- Published in Corporate News / Blog
STANFORD RESEARCHERS ISOLATE SKELETAL STEM CELLS THAT GIVE RISE TO BONES AND CARTILAGE
Stanford Researchers Isolate Skeletal Stem Cells That Give Rise to Bones and Cartilage
Cartilage and bone deterioration often result from aging, lifestyle factors, or injuries. Unlike bone tissue, mature cartilage has limited self-healing capabilities. Surgical interventions like joint replacement are costly and carry risks such as rejection and infection [1].
Stanford’s Breakthrough in Tissue Engineering
In January 2015, Stanford University School of Medicine scientists made a significant breakthrough in tissue engineering. They successfully isolated skeletal stem cells (myoblasts) capable of generating bone and cartilage in mice. This achievement included mapping the chemical signals crucial for directing stem cell differentiation [2].
Understanding Skeletal Stem Cells
Stem cells are undifferentiated cells with the ability to develop into specialized cell types, replenishing damaged tissues. While embryonic stem cells (ESCs) are pluripotent and controversial due to ethical concerns, adult stem cells from sources like bone marrow and adipose tissue are promising alternatives. Recent research suggests that adult stem cells may differentiate into diverse cell types beyond their tissue of origin.
Stanford’s Research Insights
Stanford’s study focused on rapidly dividing cells found at the ends of mouse bones. Human skeletal muscle-derived cells transplanted into mice showed remarkable regenerative potential after irradiation and cryoinjury preconditioning. This research not only reconstructed bone tissues but also mapped the developmental trajectory of skeletal stem cells.
Irving Weissman, MD, director of the Stanford Institute for Stem Cell Biology and Regenerative Medicine, anticipates these findings will advance human therapies for cartilage and bone regeneration [3].
Differentiation and Therapeutic Applications
Skeletal stem cells contribute to bone formation, adipocyte production (fat cells), and cartilage regeneration. Their potential therapeutic applications extend to treating muscular conditions like muscular dystrophy, joint disorders such as arthritis, and even autoimmune diseases like rheumatoid arthritis and multiple sclerosis.
Challenges and Future Prospects
Despite promising results, challenges in scaling up cell production remain a barrier to widespread clinical application of skeletal stem cell therapies.
References
- Development and Remodeling of Skeletal Tissue – David King, School of Medicine, Southern Illinois University, 2009
- Researchers Isolate Stem Cell That Gives Rise to Bones, Cartilage in Mice – Christopher Vaughan, Stanford Institute for Stem Cell Biology and Regenerative Medicine, 2015
- Stanford Stem Cell Research
- Genetic Control of Skeletal Development
- Published in Corporate News / Blog
PROTEINS PRODUCED BY STEM CELLS, USED IN BONE REGENERATION
Bone loss and weakening are natural effects of aging, beginning as early as age 30. Factors like smoking, inadequate calcium intake, certain medications, and hormonal changes accelerate bone resorption, leading to decreased density, porous bones, and increased fracture risk.
Stem Cell Innovations in Bone Reconstruction
Researchers at the Gladstone Institutes have pioneered a method using proteins derived from stem cells to stimulate bone reconstruction. Unlike traditional methods that rely on bone grafts from cadavers, this approach extracts bone-forming proteins directly from stem cells.
Advantages of Stem Cell Proteins
In experiments on mice, proteins extracted from stem cells were injected into muscle tissue, promoting significant bone growth without the risks associated with traditional bone grafts. This method shows promise for safer, more effective bone repair in humans, with lower rejection and tumor formation risks [1].
Scientific Validation and Clinical Applications
A study published in Scientific Reports affirmed that stem cell-derived proteins offer a reliable source for tissue regeneration. This builds on earlier research, such as Dr. Ranieri Cancedda’s pioneering work using bone marrow cells to repair bone defects [2].
Cutting-Edge Research and Future Directions
Researchers at the National Institutes of Health successfully grew new bone from stem cells derived from skin cells, published in Cell Reports. By reprogramming skin cells into induced pluripotent stem cells (iPSCs) and differentiating them into bone cell precursors, they achieved bone growth in primates without tumor formation. This method harnesses the immune compatibility and versatility of iPSCs, heralding new possibilities for personalized bone regeneration [3].
Conclusion
While the use of stem cells in bone regeneration is advancing, ongoing research aims to refine techniques for optimal integration and long-term effectiveness in human patients.
References
- Published in Corporate News / Blog
The different types of stem cells and their current uses
Stem cells possess remarkable potential in medical applications due to their ability to differentiate into diverse cell types and renew themselves. They play a crucial role in the body by generating specialized cells that form tissues such as muscles, bones, blood, and the brain.
Types of Stem Cells Based on Origin
Embryonic Stem Cells (ESCs)
Embryonic stem cells are pluripotent cells derived from embryos up to 5 days old. They can differentiate into any cell type, making them valuable for tissue repair and regeneration. Ethical considerations limit their use in research and clinical applications.
Adult Stem Cells
Found in mature tissues throughout the body, adult stem cells can differentiate into specific cell types related to their tissue of origin. Recent studies suggest they may have broader differentiation potential similar to embryonic stem cells. Adult stem cells, such as those from bone marrow, are used in therapies for bone, cartilage, and blood vessel repair.
Induced Pluripotent Stem Cells (iPSCs)
Generated from adult specialized cells through genetic reprogramming, iPSCs exhibit characteristics akin to embryonic stem cells without ethical concerns. They show promise in treating neurological disorders and other conditions, offering versatility and reduced risk of rejection compared to adult stem cells.
Current Applications of Stem Cells by Type
Adult Stem Cells
Adult stem cells from bone marrow are crucial in treating leukemia and related cancers via bone marrow transplants. They also aid in repairing cartilage defects and enhancing outcomes in spinal cord injuries and peripheral vascular disease.
Induced Pluripotent Stem Cells (iPSCs)
Research using iPSCs derived from skin cells highlights their potential in treating neurological disorders like Parkinson’s disease and age-related macular degeneration. Studies have successfully grown human liver buds from iPSCs, opening new avenues for organ regeneration and disease treatment.
Challenges and Future Directions
While adult stem cells and iPSCs offer extensive therapeutic possibilities, challenges such as immunological rejection remain significant barriers. Ongoing research aims to overcome these hurdles and expand the clinical use of stem cells in various medical fields.
For more information on stem cell research and therapies, explore our comprehensive resources and stay updated on the latest advancements in regenerative medicine at www.issca.us.
- Published in Corporate News / Blog