Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise
Introduction
The clinical demand for bioengineered blood vessels continues to rise, yet current options for vascular conduits remain limited. The synergistic combination of emerging advances in tissue fabrication and stem cell engineering promises new strategies for engineering autologous blood vessels that recapitulate not only the mechanical properties of native vessels but also their biological function. Here, we explore recent bioengineering advances in creating functional blood macro and microvessels, particularly featuring stem cells as a seed source. We also highlight progress in integrating engineered vascular tissues with the host after implantation and the exciting pre-clinical and clinical applications of this technology.
The Clinical Need for Vascular Grafts
Ischemic diseases, such as atherosclerotic cardiovascular disease (CVD), remain one of the leading causes of mortality and morbidity worldwide (GBD 2015 Mortality and Causes of Death Collaborators, 2016; Mozaffarian et al., 2016). These diseases have resulted in an ever-persistent demand for vascular conduits to reconstruct or bypass vascular occlusions and aneurysms. Synthetic grafts for replacing occluded arterial vessels were first introduced in the 1950s following surgical complications associated with harvesting vessels, the frequent shortage of allogeneic grafts, and immunologic rejection of large animal-derived vessels. Despite advances in pharmacology, materials science, and device fabrication, these synthetic vascular grafts have not significantly decreased overall mortality and morbidity (Nugent and Edelman, 2003; Prabhakaran et al., 2017).
Challenges with Synthetic Grafts
Synthetic grafts continue to exhibit a number of shortcomings that have limited their impact. These shortcomings include low patency rates for small diameter vessels (< 6 mm in diameter), a lack of growth potential for the pediatric population necessitating repeated interventions, and susceptibility to infection. Vascular conduits are also needed for clinical situations such as hemodialysis, which involves large volumes of blood that must be withdrawn and circulated back into a patient several times a week for several hours.
The Importance of Microvascularization
In addition to large-scale vessel complications, ischemic diseases also arise at the microvasculature level (< 1 mm in diameter), where replacing upstream arteries would not address the reperfusion needs of downstream tissues (Hausenloy and Yellon, 2013; Krug et al., 1966). Microvascularization has proven to be a critical step during regeneration and wound healing, where the delay of wound perfusion (in diabetic patients, for example) significantly slows down the formation of granulation tissue and can lead to severe infection and ulceration (Baltzis et al., 2014; Brem and Tomic-Canic, 2007; Randeria et al., 2015).
Structural Components of Blood Vessels
To design advanced grafts, it is important to consider the structural components of a blood vessel. Many different blood vessel beds share common structural features. Arteries, veins, and capillaries have a tunica intima comprised of endothelial cells (EC), which regulate coagulation, confer selective permeability, and participate in immune cell trafficking (Herbert and Stainier, 2011; Potente et al., 2011). Arteries and veins are further bound by a second layer, the tunica media, composed of smooth muscle cells (SMC), collagen, elastin, and proteoglycans, conferring strength to the vessel and acting as effectors of vascular tone. Vascular tissue engineering has evolved to generate constructs that incorporate the functionality of these structural layers, withstand physiological stresses inherent to the cardiovascular system, and promote integration in host tissue without mounting immunologic rejection (Chang and Niklason, 2017).
Suitable Cell Sources
A suitable cell source is critical to help impart structural stability and facilitate in vivo integration. Patient-derived autologous cells are one potential source that has garnered interest because of their potential to minimize graft rejection. However, isolating and expanding viable primary cells to a therapeutically relevant scale may be limited, given that patients with advanced arterial disease likely have cells with reduced growth or regenerative potential. With the advancement of stem cell (SC) technology and gene editing tools such as CRISPR, autologous adult and induced pluripotent stem cells (iPSCs) are emerging as promising alternative sources of ECs and perivascular SMCs that can be incorporated into the engineered vasculature (Chan et al., 2017; Wang et al., 2017).
Engineering and Integration of Vascular Tissues
A viable cell source alone is not sufficient for therapeutic efficacy. Although vascular cells can contribute paracrine factors and have regenerative capacity, merely delivering a dispersed mixture of ECs to the host tissue has shown limited success at forming vasculature or integrating with the host vasculature (Chen et al., 2010). Recent tissue engineering efforts have focused on recreating the architecture and function of the vasculature in vitro before implantation, with the hypothesis that pre-vascularized grafts and tissues enhance integration with the host.
Clinical Applications
The first reported successful clinical application of tissue-engineered blood vessels (TEBV) in patients was performed by Shin’oka et al., who implanted a biodegradable construct as a pulmonary conduit in a child with pulmonary atresia and single ventricle anatomy (Shin’oka et al., 2001). The construct was composed of a synthetic polymer mixture of L-lactide and e-caprolactone, reinforced with PGA, and seeded with autologous bone marrow-derived mesenchymal stem cells (BM-MSCs). The authors demonstrated patency and patient survival 7 months post-implant, and expanded their study to a series of 23 implanted TEBVs and 19 tissue patch repairs in pediatric patients (Hibino et al., 2010). They reported no graft-related mortality, and four patients required interventions to relieve stenosis at a mean follow-up of 5.8 years.
The first sheet-based technology to seed cultured autologous cells, developed by L’Heureux et al., induced cultured fibroblast cell sheets over a 10-week maturation period to produce tubules of endogenous ECM over a production time ranging between 6 and 9 months. They dehydrated and provided a living adventitial layer before seeding the constructs with ECs (L’Heureux et al., 2006). Their TEBV, named the Lifeline graft, was implanted in 9 of 10 enrolled patients with end-stage renal disease on hemodialysis and failing access grafts in a clinical trial. Six of the nine surviving patients had patent grafts at 6 months, while the remaining grafts failed due to thrombosis, rejection, and failure (McAllister et al., 2009).
Future Directions
Harnessing the regenerative functions reported in ECs derived from adult stem cells and iPSCs offers the promise of improving TEBV patency. Mcllhenny et al. generated ECs from adipose-derived stromal cells, transfected them with an adenoviral vector carrying the endothelial nitric oxide synthase (eNOS) gene, and seeded the ECs onto decellularized human saphenous vein scaffolds (McIlhenny et al., 2015). They hypothesized that through inhibition of platelet aggregation and adhesion molecule expression, nitric oxide synthesis would prevent thrombotic occlusion in TEBV. Indeed, they reported patency with a non-thrombogenic surface 2 months post-implantation in rabbit aortas. Engineering ECs and SMCs with other regenerative, anti-inflammatory, and anti-thrombotic genes could bridge the functional difference between SC-derived cells and native primary cells.
- Published in Corporate News / Blog
How does the U.S. FDA regulate cell therapies?
Cell therapies in the United States are regulated by the FDA’s Office of Cellular, Tissue, and Gene Therapies (OCTGT), part of the FDA Center for Biologics Evaluation and Research (CBER). Understanding these regulations is crucial for companies involved in the development and distribution of cellular products.
Overview of FDA Regulation
The FDA’s Center for Biologics Evaluation and Research (CBER) oversees:
- Cellular therapy products
- Human gene therapy products
- Certain devices related to cell and gene therapy
CBER operates under the authority of the Public Health Service Act and the Federal Food Drug and Cosmetic Act, which provide the legal framework for regulatory oversight.
Regulation Pathways: 351 vs. 361 Products
361 Products
Products that meet all criteria outlined in 21 CFR 1271.10(a) are classified as Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) under Section 361 of the Public Health Service (PHS) Act. These products, known as “361 products,” do not require FDA licensure or approval.
351 Products
Products that do not meet all criteria outlined in 21 CFR 1271.10(a) are regulated under Section 351 of the PHS Act and the Federal Food, Drug, and Cosmetic Act. These products are treated as drugs, devices, or biological products and require FDA approval through clinical trials to demonstrate safety and efficacy.
Regulatory Framework
The distinction between 361 and 351 products reflects the FDA’s assessment of relative risk associated with each type of cell therapy. 361 products are considered lower risk and are subject to less stringent regulatory requirements compared to 351 products, which undergo a rigorous approval process akin to pharmaceuticals.
Conclusion
Navigating FDA regulations for cell therapies involves understanding whether a product falls under the 361 or 51 pathway. Compliance with these regulations is essential for ensuring safety, efficacy, and legal market entry for cell therapy products in the United States.

- Published in Corporate News / Blog
What is regenerative medicine?
Regenerative medicine is the cutting-edge “medicine of the future,” offering the promise of efficacy by enabling human tissue to be repaired, replaced, and healed (regenerated) once it is damaged or diseased. These therapies supplement the body’s natural healing mechanisms, often employing stem cells to stimulate the renewal of tissue damaged by injury, disease, or age. The rapid expansion of scientific knowledge offers great promise for advances in this field, holding vast potential to improve the quality of human life.
What are Stem Cells?
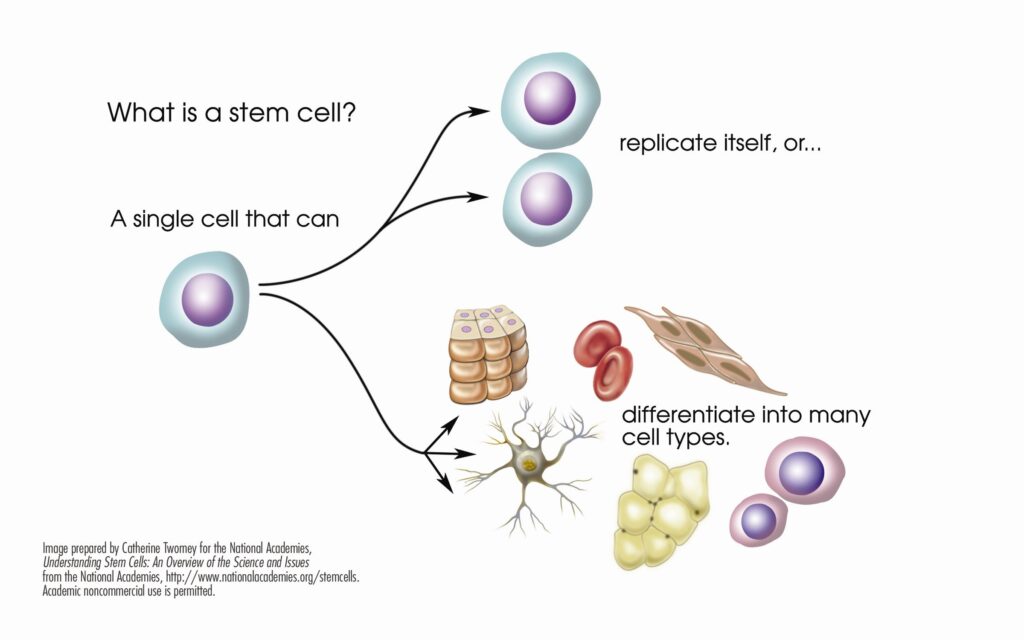
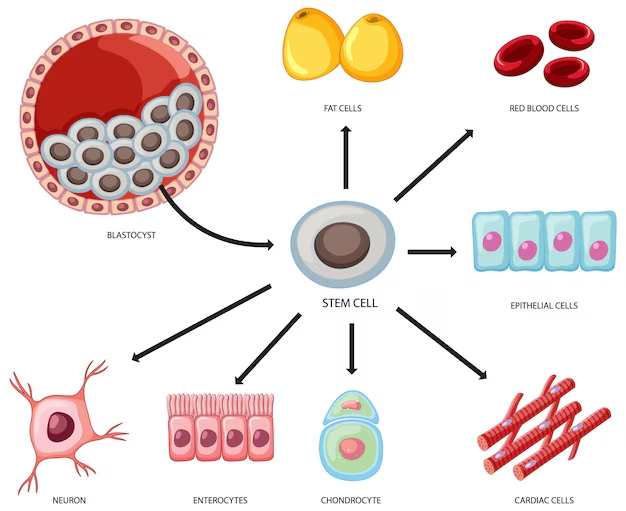
Stem cells are the basic building blocks of life. They are unspecialized cells capable of producing more stem cells through mitosis or differentiating into specialized cells that perform specific functions in the body. Stem cells are found throughout the body’s tissues, organs, and systems, although usually in small quantities in adults.
What are Hematopoietic Stem Cells?
Hematopoietic stem cells (HSCs) can give rise to all types of blood cells, including red blood cells, white blood cells, and platelets. They are particularly useful in treating blood-related diseases and conditions.
What are Mesenchymal Stem Cells?
Mesenchymal Stem Cells (MSCs) are multipotent stromal cells that are non-blood forming stem cells capable of differentiating into a variety of cell types, including muscle, bone, cartilage, and fat cells. When introduced into a patient’s body, MSCs can repair or replace damaged or degenerating tissue by communicating with surrounding cells, triggering a cellular cascade of healing (paracrine signaling).
The term MSC was coined in the late 1980s by Dr. Arnold Caplan of Case Western Reserve. Recently, Caplan redefined the acronym to “Medicinal Signaling Cell” to emphasize these cells’ role in secreting powerful bioactive molecules involved in cellular signaling and regeneration. Caplan now describes MSCs as a “multisite-regulatory dispensary” (Natural Drug Store).
The production of MSCs in the human body can be precipitated by bioactive placental tissues containing Growth Factors, Cytokines, and other powerful bioactive agents that trigger cell signaling. The remarkable ability of MSCs makes them irreplaceable in medical treatments.
What are Accessible Sources of Stem Cells?
Stem cells can be extracted from various parts of the body, such as bone marrow and adipose tissue. More recently, birth tissues from live births, including umbilical cord blood, cord tissue with Wharton’s Jelly, and amniotic membrane tissue, have been found to be rich sources of both HSCs and MSCs. These tissues also precipitate the production of MSCs through paracrine signaling. Growth Factors, Cytokines, Exosomes, and micro-RNA from birth tissues give rise to stem cells in this way. These cells, as well as MSCs contained in Wharton’s Jelly, tend to be more fit than those obtained from adult stem cells.
Wharton’s Jelly
Wharton’s Jelly is a gelatinous substance found in the umbilical cord that is rich in stem cells. Studies have shown that mesenchymal stem cells (MSCs) derived from Wharton’s Jelly have low immunogenicity. Human umbilical cord Wharton’s Jelly provides a new source for MSCs that are highly proliferative and have multi-differentiation potential. Wharton’s Jelly Cells (WJCs) express MSC markers but have low levels of human leukocyte antigen (HLA)-ABC and no HLA-DR. WJCs have low functional immunogenicity, and recipient rejection has not been documented.
Advanced Regenerative Medicine
Advanced Regenerative Medicine involves the use of regenerative biomolecules, tissue engineering, and stem cells to treat diseased or injured tissues. This field leverages cutting-edge techniques to promote healing and regeneration, offering new hope for patients with previously untreatable conditions.
- Published in Corporate News / Blog
Controlling Stem Cells… through their environment
Stem cells have captured the imagination of both scientists and the public alike with their potential to revolutionize medical treatments and therapies. The ability of these microscopic entities to differentiate into various cell types holds promise for curing diseases and repairing damaged tissues.
Understanding Stem Cell Differentiation
Researchers are actively exploring methods to manipulate stem cell development, particularly pluripotent cells capable of becoming any cell type. By culturing these cells in specific environments, scientists have observed activation of genes that dictate tissue specialization. This insight is crucial for developing targeted therapies and treatments.
Innovations in Stem Cell Research
Recent breakthroughs include the generation of dopamine-producing neurons from pluripotent cells and the creation of sugar-based scaffolds for blood vessels. These advancements highlight the potential of stem cells to address complex medical challenges.
Clinical Applications of Adult Stem Cells
Physicians have increasingly utilized adult stem cells sourced from fat or bone marrow to treat degenerative conditions such as knee osteoarthritis, sports injuries, and frozen shoulder. Unlike pluripotent cells, adult stem cells primarily function by releasing growth factors, proteins, and anti-inflammatory substances that promote tissue regeneration and healing.
Natural Healing Through Cellular Environment
In clinical settings, adult stem cells influence their environment by enhancing local cell growth and differentiation. This natural process mimics laboratory findings, demonstrating the body’s innate ability to harness its own healing potential.
Conclusion
The field of stem cell research continues to evolve, promising new avenues for medical intervention and patient care. By understanding how stem cells interact with their environment, researchers and healthcare providers can optimize therapies and explore novel treatments for a range of conditions

- Published in Corporate News / Blog
Photobiomodulation and Why We Use LED to Irradiate PRP
Introduction to PRP in Regenerative Medicine
In the regenerative medicine specialty, we have been active for about 8 years. Like many others, we started by using Platelet-Rich Plasma (PRP). We learned about it, were fascinated by it, and treated our patients with it. Patients loved it, and so did we.
PRP, if obtained and used correctly, is a powerful tool to implement in any medical practice, especially when dealing with the elderly, osteoarthritis, wear and tear of tendons and ligaments, and loss of vitality. PRP is also frequently used in plastic and reconstructive surgery for wound care, scar improvement, and overall skin rejuvenation.
How PRP Works
But why does PRP work, and how?
We are all familiar with platelets. They have significant power and influence over tissue regeneration, and by concentrating them in a blood sample, we can obtain signaling proteins, cytokines, and growth factors. If we add white blood cells to the mix, we get what is called L-PRP (leukocyte-rich PRP), making that “soup” even more interesting.
What is Photobiomodulation?
To harness the power of these bioactive substances, we need to coax the cells into releasing them. Normally, platelets are activated by the addition of calcium or by contact with collagen. However, several studies have demonstrated the influence of low-intensity laser on the activity of some cells. This effect is called “photobiomodulation.” This modality uses non-ionizing light sources, like LEDs or Helium-Neon lasers, to produce photochemical events at various biological scales. It has been demonstrated that this light reacts with the enzyme cytochrome c oxidase, which is paramount in mitochondrial processes.
Effects of Photobiomodulation on Cellular Cultures
Several scientists studied this light and its effects on cellular cultures. They found that cells proliferate more when exposed to low-level laser and showed increased viability.
We compared the levels of cytokines and growth factors in an irradiated sample and a non-irradiated one. Some growth factors even tripled in concentration after laser exposure. The famous interleukin-10, an anti-inflammatory protein, doubled in levels, and endorphins were released in high levels.
Benefits of Photobiomodulation
The photobiomodulation process has been established to provide extraordinary benefits in pain management, inflammation reduction, immunomodulation, and promotion of wound healing and tissue regeneration. It plays a fundamental part in our protocols.
Isn’t it all sort of amazing?
We’ll see you in the next blog. Keep your cells healthy!
- Published in Corporate News / Blog
Top 3 Reasons We Offer Doctors Marketing Services
Introduction
At Adimarket, we sell equipment to practices that are willing and able to add PRP and stem cell therapies to their lineup. The equipment we offer is among the best, and we have helped hundreds of doctors and practices to offer PRP and stem cell therapies. However, we also provide marketing services as well.
Although it might seem odd that we offer both marketing services and equipment, it makes sense once you understand why. Simply offering services and having the equipment to do so does not in itself help patients fully know that you are offering new services. It is best practice to get the word out to as many people in the area as possible.
Top 3 Reasons We Offer Doctors Marketing Services

1. Regenerative Medicine Is Relatively New
Compared to many other medical practices, such as surgery and physical therapy, regenerative medicine is still fairly new. In fact, most people do not really know that PRP and stem cell therapy even exist, let alone that they can be used to manage chronic pain.
The fact that not many people even know about the existence of regenerative medicine, let alone what it can be used for, means that it would be difficult to get your patients to even understand what you are offering as a service. This can be addressed with marketing. Through marketing, a practice can not only make it known that they are offering these new services, but also explain what the service entails.
2. Marketing Is Like Dieting
Pretty much every doctor and dietitian knows that good nutrition is vital to great health down the line. Waiting until you’re sick and deficient to discuss nutrition is not the best way to address the issue. Marketing is similar in that instance. Marketing can not only be used to keep current patients informed but also to inform new patients about what you offer. Practices that don’t market often suffer in the same way as people who don’t get good nutrition.
3. There’s a Lot of Competition
Medicine has sadly become more and more like a business in recent years. This means that even doctors and practices need to have a good business sense if they are going to continue to provide the type of services that patients need and desire. Not understanding business would only make any practice fail or at least prevent them from growing.
Because of this, private practices, as well as other medical groups, are forced to compete. Marketing is a big way to make sure that you get patients instead of your competition. If you are utilizing PRP and stem cell therapies as a way to generate more income, then great! However, you will still have to market those services to get the word out and compete.
Conclusion
At Adimarket, we offer these services to help the field of regenerative medicine succeed. We not only help your practice start to utilize regenerative medicine, but we also help you promote your practice. This will help your patients know that you are using these methods and what they are, so that you can get a leg up over the competition.
- Published in Corporate News / Blog
Stem Cell Therapy’s Future
Stem cells from adults revolutionized leukemia treatment through bone marrow transplants over 30 years ago. This success spurred scientists to explore broader applications of stem cell therapy for various diseases and injuries. While initial expectations may have been lofty, the field continues to evolve with promising advancements on the horizon.

What Are The Different Types of Stem Cells?
Early research primarily focused on two types of stem cells: Adult and Embryonic. Adult stem cells, notably used in bone marrow transplants, showed efficacy in treating several conditions. In contrast, embryonic stem cells, although versatile, posed practical challenges that limited their widespread use.
In 2006, Japanese researchers pioneered a breakthrough by reprogramming adult stem cells into induced Pluripotent Stem (iPS) cells, mimicking embryonic stem cells’ versatility. However, concerns emerged over iPS cells’ susceptibility to mutations, leading to occasional cancer risks.
Making The Research More Targeted
Despite setbacks, these challenges deepened our understanding of stem cell biology. Current research focuses on targeted therapies tailored to specific tissue damage, marking a significant shift in strategy. Unlike earlier methods reliant on cell reprogramming, today’s approach emphasizes using tissue-specific stem cells for precise applications.
Stem cells derived from joint tissues, for instance, can regenerate only joint tissues, highlighting current limitations. Yet, these constraints are viewed as temporary barriers that ongoing research is poised to overcome.
Adimarket’s Role in Advancing Stem Cell Therapies
At Adimarket, we equip medical practices with the tools needed for Platelet Rich Plasma (PRP) and Stem Cell therapies. Our goal is to expand treatment options for patients suffering from injuries and arthritis, contributing to the bright future of regenerative medicine.
PRP and stem cell therapies hold immense potential for the future of medical treatment. Medical professionals and practice owners can join this transformative journey by integrating our cutting-edge equipment into their practices. For more information or inquiries, visit our website today.
- Published in Corporate News / Blog
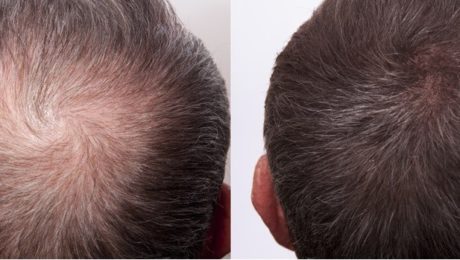
PRP and Stem Cell Treatments Being Studied for Hair Loss
Hair loss is a widespread issue, affecting tens of millions of people worldwide. Many individuals turn to hair restoration therapies and other procedures to regain their lost hair. While some treatments are effective, they often merely relocate existing hair. Stem cell therapy, however, aims to stimulate new hair growth by regenerating hair follicles.
The Promise of PRP and Stem Cell Therapies
At Adimarket, along with other innovative companies, we are dedicated to exploring and providing reliable uses for PRP (Platelet-Rich Plasma) and stem cell therapies. We supply the necessary equipment and services to enable doctors and practices to offer these advanced treatments to their patients.
Leading Experts and Ongoing Studies
Although smaller companies like ours are at the forefront of discussing PRP and stem cell therapies, many in the medical community see the potential in these treatments. Notable practitioners, such as Dr. Lazaro M Garcia from Miami, are already utilizing PRP and stem cell therapy for hair loss. Dr. Garcia is currently conducting a study supported by the National Institutes of Health.

How the Study Works
Patients participating in the study pay a small fee, varying by their study group. They receive two injections of PRP and stem cells derived from their own body over three months. Dr. Garcia’s approach leverages the body’s own growth factors to enhance blood flow and nutrient delivery to dormant hair follicles, revitalizing them and promoting new hair growth.
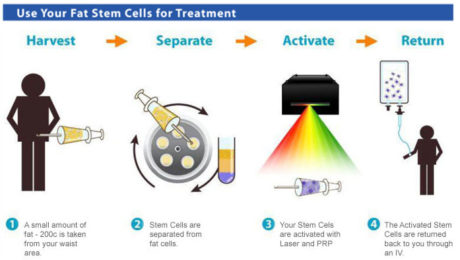
The Process of Creating Platelet-Rich Plasma
Creating PRP involves using the patient’s own blood, which can be sourced from bone marrow or other fat deposits. The blood is processed in a centrifuge to concentrate its components, which are then injected into the treatment site. Although the procedure is straightforward, proper training is essential to ensure safety and efficacy.
Adimarket’s Role in Providing Equipment
At Adimarket, we don’t perform stem cell and PRP therapies ourselves, but we offer the equipment necessary for doctors and practices to do so. Our products include amniotic tissue, stem cell and PRP kits, centrifuge devices, and more. Practices and doctors can conveniently order directly from us.
Superior PRP Systems
It’s important to note that not all PRP systems are created equal. Our closed tabletop system can process PRP in under 10 minutes, ensuring efficiency and reliability. For more information, contact us or visit our website.
Conclusion
Hair loss is a significant concern for many people. Thanks to advancements in PRP and stem cell therapies, we no longer have to rely solely on hair replacement surgery. These therapies could be the most effective solutions for both patients and medical practices, offering new hope for those suffering from hair loss.
- Published in Corporate News / Blog
Why Do We Side With Stem-Cell Therapy?
PRP and Stem Cell Therapy still have a ways to go before becoming mainstream medical practices. However, we at Adimarket are proud to be part of this growing trend. We fervently believe in the future of stem cell therapies because we trust that they offer an alternative to otherwise invasive and unnecessary medical procedures.
If we listed every single scientific reason showing the benefits of stem cell therapy, we would be writing a book. To keep it concise, we have decided to highlight three main reasons why we support this therapy and sell equipment for it.
1. It’s Been Around for a While
Stem cell therapy is neither a new nor an untested invention. This therapy has been used for decades in various procedures, such as bone marrow transplants for leukemia patients. The main issue is that stem cell therapy is not widely known.
This therapy has benefited people suffering from leukemia and other diseases like lymphoma for decades, primarily through bone marrow transplants. The safety and minimal risk of rejection, as stem cells are the body’s own, make this therapy a viable option. If they can be used to treat these conditions, there is likely much more they can do.
2. It’s Not Really Invasive
While invasive procedures will probably never be entirely obsolete (e.g., bone fractures or tooth extractions), not all invasive surgeries are necessary.
At Adimarket, we love stem cell therapy because it is minimally invasive. Instead of requiring incisions and stitches, the most a patient needs is a needle poke to extract stem cells and another to inject them into the treatment area. This reduces the risk of infection and other complications, such as scarring.
3. It Works to Improve Healing
The final reason we promote stem cell therapy is that it allows the body to heal naturally. This means that the treatment addresses the underlying problem rather than just focusing on symptom management.
We hope this approach helps patients receive better and more effective treatment, addressing the entire issue instead of just the symptoms. While stem cell therapy might not be the best choice for every case, it is similar to any other medical procedure. Not all treatments require medication and surgery.
Why Do We Side With Stem-Cell Therapy?
If you seek better and more efficient ways of treating injuries and various diseases, Adimarket is the place to go. We provide the latest and greatest technology and equipment related to PRP and stem cell therapy, helping you find better treatments for your patients.
Stem cell therapy can be highly beneficial not only for treating patients but also for increasing your revenue. Incorporating stem cell therapy may very well be the best thing you can do for your practice.
- Published in Corporate News / Blog
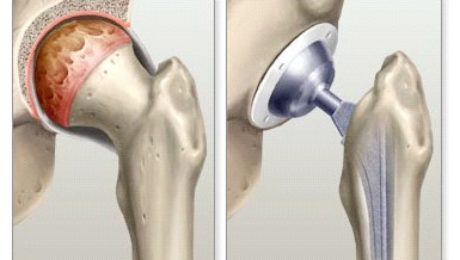
Can Hip Replacements Be Obsolete Through The Use Of Stem Cell Therapy?
As more and more doctors train to be proficient in stem cell therapy, many seek to increase their revenues and expand their practices. At Adimarket, we aim to fulfill the needs of doctors and help their patients in the most efficient way possible. However, we have other goals as well. We hope that one day, PRP and stem cell therapies will replace certain medical procedures, making them more effective and convenient. One such procedure we hope to eliminate is hip replacements.
Natural Tissue Regeneration in Orthopedics
Natural tissue regeneration has been a focus for many scientists for years, particularly in orthopedics, the study of bones and muscles. Utilizing a patient’s stem cells to regrow or replace cartilage can be a godsend for treating conditions such as osteoarthritis, as well as sports and other injuries. Many wonder if we can heal muscle and joint damage using PRP and stem cell therapy, just how much more can we potentially heal?
Potential of Stem Cell Therapy Over Hip Replacement Surgery
Based on current evidence, it may be possible in the near future to consider PRP and stem cell therapy over hip replacement surgery. A 2016 study published in the Proceedings of the National Academy of Sciences showed that stem cells successfully grew on an artificial hip joint. The researchers even created a therapy that, combined with stem cells, could lower the risk of arthritis recurrence in the joint.
What Treatments Are We Already Utilizing?
While seeing hip replacements become a thing of the past is a potential occurrence in the near future, the science is not there yet. Adimarket is working to provide the tools necessary to utilize both PRP and stem cell therapy in treating sports injuries, wounds, and orthopedic applications.
In fact, the two therapies are in no way mutually exclusive. In many cases, both PRP and stem cell therapies can be utilized simultaneously, as long as the stem cells come from the patient themselves. This helps reduce the risk of rejection.
PRP can be extracted from the blood, and the stem cells can come from bone marrow or fat tissue. These are then injected directly into the treatment site, and we simply wait for the magic to happen.
Adimarket Has Got You Covered
If you are a doctor or own your practice, we urge you to consider adding PRP and stem cell therapies to your treatment options. Adimarket provides a wide range of technology and equipment designed to give patients the best experience and treatment possible.
Our equipment is some of the best in the trade. We provide fast and reliable processing of platelets, capable of concentrating blood platelets up to 16 times. If you are interested in learning more or providing this equipment in your practice, consider looking up Adimarket.
Will we ever see hip replacement surgery become obsolete? Possibly. It is our hope that it does. Until then, we aim to provide the necessary technology to help doctors offer the best stem cell and PRP treatments to their patients.
ide the necessary technology to help doctors provide the best stem cell and PRP treatment to their patients.
- Published in Corporate News / Blog