Global Stem Cells Group and Santiago University to Launch New Edition of Post-Graduate Diploma Program
MIAMI, April 30, 2015–Global Stem Cells Group and the University of Santiago, Chile have announced plans to launch a new edition of the post-graduate diploma program, “Diplomat in Cell Therapy and Tissue Engineering.” The first of its kind worldwide, the program is designed for physicians and qualified practitioners to bring stem cell therapies into the doctor’s office to treat patients.
The University of Santiago, ranked among the top universities in South America, is the first state university to offer a diploma program in stem cells, tissue engineering and cell therapy.
The program concentrates on advances in cell biology, the clinical and linked characteristics to bioprocessing of stem cells derived from adipose tissue, and other stem cell-based protocols. Based on medical research that emerged in the late 20th and early 21st centuries to give rise to stem cell therapies, this is a new form of medical treatment in which cells and tissues are used as healing elements, not only to supplement or replace deficient cells, but to induce regeneration and restoration of a lost biological order during the development of a disease or injury.
 Alejandra Moenen, Ph.D., who heads the University of Santiago’s Biochemistry and Molecular Biology Department, was chosen to teach the program. Moenen is an internationally prominent researcher whose work in biological research has been published in 50 major scientific journals worldwide.
Alejandra Moenen, Ph.D., who heads the University of Santiago’s Biochemistry and Molecular Biology Department, was chosen to teach the program. Moenen is an internationally prominent researcher whose work in biological research has been published in 50 major scientific journals worldwide.
Global Stem Cells Group has established the program’s medical and scientific management team, headed by Duncan Ross, Ph.D. and Enrique Testart, M.D., to develop a theoretical and practical, 120 hour program that will deliver the fundamentals of the most advanced cell therapies and clinical applications currently practiced safely and effectively in more than 35 cities worldwide.
In a 2015 televised interview, Joseph Purita, M.D., an orthopedic surgeon, stem cell pioneer and founder of the American Academy of Regenerative Medicine, said that Santiago University has all the human and physical resources and conditions necessary to become the capital of stem cell research and advances in South America.
Purita’s foresight launched an alliance between GSCG and Santiago University that led way to building an academic program to meet the needs of physicians, engineers, biologists and biochemists required to move regenerative medicine forward.
 The Diplomat in Cell Therapy and Tissue Engineering program will begin in 2016, with a first phase designed to carry it through 2020. A maximum of 15 students will make up groups who will occupy individual biological work stations, study electron microscopy, flow cytometry, real-time polymerase chain reaction (PCR) and faculty-arranged resources to make this international diploma program a recognized leader worldwide for the training of specialists in the field of stem cell medicine.
The Diplomat in Cell Therapy and Tissue Engineering program will begin in 2016, with a first phase designed to carry it through 2020. A maximum of 15 students will make up groups who will occupy individual biological work stations, study electron microscopy, flow cytometry, real-time polymerase chain reaction (PCR) and faculty-arranged resources to make this international diploma program a recognized leader worldwide for the training of specialists in the field of stem cell medicine.
Open enrollment will begin April 30 for high-achieving medical professionals with qualified credentials, who will receive dual certification—a diploma in Cell Therapy and Tissue Engineering from the University of Santiago, and the American Certificate of Protocols from Global Stem Cells Group, and its affiliate, Stem Cell Training, Inc. The course will include practical, hands-on training during which students will apply stem cell harvesting and implantation techniques learned in the laboratory, quantify cell counts, weigh comparisons and ultimately experience stem cell therapy in all its dimensions, to use in different areas of medicine and dentistry.
“Between physicians, biologists, biochemists and engineers, we have collectively built a curriculum that is designed for the new generation of medicine,” says Testart, Global Stem Cells Group Chief Medical Officer. “But even more important, we will provide a new generation of patients with therapies that will enable them to improve their quality of life for years to come, and to enjoy the longevity given us in the modern world.”
“The Diplomat in Cell Therapy and Tissue Engineering program will offer medical professionals invaluable lessons in this new art of stem regenerative healing, as well as the scientific and practical methodologies involved in stem cell medicine,” says Benito Novas, Global Stem Cells Group CEO.
Educational strategies will be taught in theory and in practical hands-on classes, during which students can raise questions and work on problem solving.
For more information, visit the Global Stem Cells Group website, email bnovas(at)stemcellsgroup(dot)com, or call +1 305 560 5337.
About Global Stem Cells Group:
Global Stem Cells Group is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
###
- Published in Press Releases
Global Stem Cells Group and University of Santiago Biotech Lab Endorse Asia-Pacific Symposium
Global Stem Cells Group and the University of Santiago, Chile have endorsed an Asian-Pacific alliance for a regenerative medicine and stem cell symposium July 1-2 at the university’s Santiago campus and other stem cell protocol management initiatives. Through the alliance, the two organizations established a working agenda for collaborative initiatives in stem cell and regenerative medicine research and development for 2016 – 2020.
Global Stem Cells Group and the University of Santiago Biotechnology Lab have announced a mutual endorsement of an Asia-Pacific Symposium as other research and development initiatives for potential stem cell protocol management for 2016 – 2020.
 In 2015, University of Santiago officials and top Global Stem Cells Group executives began meeting to establish a working agenda and foster initiatives to promote stem cell research and development as a collaborative effort.
In 2015, University of Santiago officials and top Global Stem Cells Group executives began meeting to establish a working agenda and foster initiatives to promote stem cell research and development as a collaborative effort.
Professor Alejandra Moenen, Ph.D., who heads the University of Santiago’s Biochemistry and Molecular Biology Department, and a team of Ph.D.s from the university will join Global Stem Cells Group for their first joint venture, an Asia-Pacific Symposium on stem cell and regenerative medicine July 1-2, 2016. Moenen is an internationally prominent researcher whose work in biological research has been published in 50 major scientific journals worldwide.
The symposium will focus on regenerative medicine and stem cell applications to anti-aging and aesthetic medicine. University of Santiago faculty will lead the symposium, which will host qualified academic and medical groups from around the world who will present their scientific papers.
Global Stem Cells Group and the University of Santiago’s Biotechnology Department decided to join forces and create a collaborative agenda based on the synergy between the two organizations.
“Chile is a country where we have first world science, without being part of developed countries,” says Moenen. “Today  we are proud to start an alliance through which we can work hand in hand with Global Stem Cells Group and its international network, which has been able to harness science to improve the quality of life for people. “
we are proud to start an alliance through which we can work hand in hand with Global Stem Cells Group and its international network, which has been able to harness science to improve the quality of life for people. “
Enrique Testart, M.D., Chief Medical Officer of Global Stem Cells Group, says he was honored to learn that a Chilean
University had initiated this new approach to collaborating with GSCG, which he believes will offer unparalleled opportunities for exchange, relationships with other institutions, and all the technology that Global Stem Cells Group can offer for studies and analysis in the area of regenerative medicine.
“A range of criteria and this innovative university are what stand out in this framework agreement,” Testart says. “It places us above any attempt to trivialize the issue.
Enrique Testart, M.D.
“Stem cells are not a fad, there are those who have been working for two decades in this field, and therefore the union between this esteemed university and this young and talented biotech company is good news for the country, for the world and for science—everyone should applaud.”
A meeting to confirm the Asia-Pacific Symposium alliance was attended by Kevin Maisey, Ph.D., and Jorge LaPorte, Ph.D., both representing the Biology and Biochemistry Department of the University of Santiago. University Dean Silvia Ferrada Vergara has validated the agreement, which will be announces at the Asia-Pacific Conference in July.
For more information, visit the Global Stem Cells Group website, email bnovas(at)stemcellsgroup(dot)com, or call +1 305 560 5337.
About Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About the University of Santiago:
Celebrating the 166th anniversary of its founding in 2016, the University of Santiago is one of the oldest and most traditional institutions of higher education in Chile. Offering 66 comprehensive undergraduate programs to more than 18,000 students, the university has seven faculties representing departments of Engineering, Humanities, Science, Business and Economics, Chemistry and Biology, Medical Sciences and Technology. The university us moving toward a new era of implementing improved and advanced master’s degree and doctoral degree programs, in addition to the numerous courses and postgraduate programs already in place in a variety of academic and research disciplines.
Since Chile’s 1981 higher education reform, the University of Santiago has concentrated its activities in the metropolitan area, with a particular focus on teaching, research and extension, carried out on the 34-hectare (84 acre) campus in the City of Santiago.
The University of Santiago is known for its participation in national and international projects and the contributions of its scholars to various fields of knowledge. A singular effort has been placed on linking the work of university researchers, who have a close relationship with the socio-economic needs of the country, to improve public health conditions in the country. The University of Santiago is one of Chile’s four Universities noted for successful fundraising efforts to support research and development.
###
To view this press release online, click here
- Published in Press Releases
Duncan Ross, Ph.D., to Speak at Santiago, Chile Symposium
Duncan Ross, Ph.D., founder of Kimera Research Labs, will be the keynote speaker at the Global Stem Cells Group Symposium in Santiago, Chile July 1-2, 2016.
 MIAMI, April 26, 2016–Global Stem Cells Group has announced that affiliate Kimera Research Labs founder Duncan Ross, Ph.D., will be the keynote speaker at the Asia-Pacific Symposium in Santiago Chile, July 1-2, 2016. The abstract for Ross’s lecture will be, “The mechanism of action of stem cells in regenerative medicine is increasingly being understood to be effected through paracrine factors. Central to the question of when and how to treat an individual disease is where and for what duration a transplanted cell will persist to generate these factors.”
MIAMI, April 26, 2016–Global Stem Cells Group has announced that affiliate Kimera Research Labs founder Duncan Ross, Ph.D., will be the keynote speaker at the Asia-Pacific Symposium in Santiago Chile, July 1-2, 2016. The abstract for Ross’s lecture will be, “The mechanism of action of stem cells in regenerative medicine is increasingly being understood to be effected through paracrine factors. Central to the question of when and how to treat an individual disease is where and for what duration a transplanted cell will persist to generate these factors.”
In the absence of a robust ability to track cell persistence in humans, Dr. Ross will present current research in murine hematopoietic and mesenchymal stem cell transplantation with support from human transplant results.

Duncan Ross, Ph.D.
The symposium will be co-sponsored by Global Stem Cells Group and the University of Santiago’s Biochemistry and Molecular Biology Department, and will focus on regenerative medicine and stem cell applications to anti-aging and aesthetic medicine. University of Santiago faculty will lead the symposium, which will host qualified academic and medical groups from around the world who will present their scientific papers.
The symposium is the first joint endeavor between Global Stem Cells Group and the University of Santiago since establishing an alliance recently, and which will be announced at the Asia-Pacific Symposium. It also marks Ross’s first appearance as a member of the Global Stem Cells Group Advisory Board.
Ross received a Ph.D. in Immunology from the University of Miami and specializes in research, mesenchymal stem cell applications, hematopoietic stem cell transplantation for hematologic disorders, the suppression of graft vs. host disease, and var
ious methods of immune suppression.
Global Stem Cells Group and Kimera Labs share a commitment to research and development, and providing stem cell treatments to patients in clinical settings worldwide.
To learn more, visit the Global Stem Cells Group website, email bnovas(at)stemcellsgroup(dot)com, or call +1 305 560 5337.
About Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About Kimera Labs:
Kimera Labs is currently focused on the use of mesenchymal stem cells (MSCs) for the suppression of various immune mediated pathologies and regenerative medicine in the US, Latin America, and the Bahamas. Founder Duncan Ross, Ph.D., is an immunologist and researcher who has studied hematopoietic stem cell transplantation for hematologic disorders, the suppression of graft vs. host disease, and various methods of immune suppression.
Kimera Labs provides patients access to stem cell treatment in the U.S. according to U.S. laws. In order to provide the greatest benefit to patients, Ross frequently travels to treat patients in Central and South America where specialists are available in a different regulatory environment.
###
To view this press release online, click here
- Published in Press Releases
Global Stem Cells Group and Kimera Labs Announce Autologous Stem Cell Research Alliance
Global Stem Cells Group and Kimera Research Labs have announced an alliance to conduct scientific research on highly manipulated cells and culture expansion, and cryopreservation of autologous stem cells.
MIAMI, April 26, 2016–Global Stem Cells Group and Kimera Research Labs have announced an alliance to conduct  scientific research on highly manipulated stem cells and culture expansion, and cryopreservation of autologous stem cells. The collaboration will open new opportunities for GSCG to increase its participation in scientific research and development of new stem cell protocols and treatments for a number of conditions.
scientific research on highly manipulated stem cells and culture expansion, and cryopreservation of autologous stem cells. The collaboration will open new opportunities for GSCG to increase its participation in scientific research and development of new stem cell protocols and treatments for a number of conditions.
The manipulation of stem cells involves the ability to deliver molecules into adherent cells without disrupting differentiation, a process biotechnology researchers need in order to advance both fundamental knowledge and the state-of-the-art in stem cell research. Differentiation is the process by which an unspecialized cell, such as a stem cell, becomes specialized into one of the many cells in the body. During differentiation, certain genes become activated and other genes become inactivated in an painstakingly regulated manner. As a result, a differentiated cell develops specific structures and performs certain functions that ultimately allows it to replace damaged or dead cells. In the laboratory, a stem cell can be manipulated to become specialized or partially specialized cell types, such as heart muscle, nerve, or pancreatic cells.
“Non-destructive manipulation of stem cells in the correct environment is key to enabling technology needed within the biology and medical research communities,” says Benito Novas, CEO of Global Stem Cells Group. “To realize the promise of stem cell-based therapies to treat injuries and diseases, scientists must be able to manipulate stem cells so that they possess the necessary characteristics for successful differentiation, transplantation, and engraftment.”
To bring successful new treatments to the clinic, scientists need to control certain steps for stem cells to be useful for transplant purposes. Researchers are constantly discovering new ways to manipulate stem cells to be reproducibly made to:
- Replicate extensively and generate sufficient quantities of cells for making tissue.
- Differentiate into the desired cell type(s).
- Survive in the recipient after transplant.
- Integrate into the surrounding tissue after transplant.
- Function appropriately for the duration of the recipient’s life.
- Avoid harming the recipient in any way.
Scientists are also experimenting with different research strategies to generate tissue without the concern of immune rejection.
 Research on cryopreservation of autologous stem cells is necessary for cell bank procedures in which stem cell expansion and use are not immediately needed. Cryopreservation allows for the long-term storage of hematopoietic stem cells (HSCs) and is the preferred storage technique for virtually all components intended for autologous HSC transplantation.
Research on cryopreservation of autologous stem cells is necessary for cell bank procedures in which stem cell expansion and use are not immediately needed. Cryopreservation allows for the long-term storage of hematopoietic stem cells (HSCs) and is the preferred storage technique for virtually all components intended for autologous HSC transplantation.
Cryopreservation allows the administration of multiple-day transplant conditioning regimens as well as elective storage for patients to receive transplants at a subsequent point in a course of treatment, and offers patients the opportunity to benefit from multidose protocols.
Global Stem Cells Group and Kimera Labs share a commitment to research, develop and provide stem cell treatments to patients worldwide in a clinical setting.
To learn more, visit the Global Stem Cells Group website, email bnovas(at)stemcellsgroup(dot)com, or call +1 305 560 5337.
About Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About Kimera Labs:
Kimera Labs is currently focused on the use of mesenchymal stem cells (MSCs) for the suppression of various immune mediated pathologies and regenerative medicine in the US, Latin America, and the Bahamas. Founder Duncan Ross, Ph.D., is an immunologist and researcher who has studied hematopoietic stem cell transplantation for hematologic disorders, the suppression of graft vs. host disease, and various methods of immune suppression.
Kimera Labs provides patients access to stem cell treatment in the U.S. according to U.S. laws. In order to provide the greatest benefit to patients, Ross frequently travels to treat patients in Central and South America where specialists are available in a different regulatory environment.
###
To view this press release live on line, click here
- Published in Press Releases
Stem Cell Researchers Discover Stem Cells That Might Repair Skull, Face Bones
Breakthrough in Stem Cell Research for Bone Repair
Scientists have made significant progress in using stem cells to potentially replace damaged skull and facial bones, crucial for patients recovering from head trauma or undergoing reconstructive surgery after cancer treatments.
Discovery of Skull and Facial Bone Repair Capabilities
Researchers at the University of Rochester Medical Center, led by Takamitsu Maruyama, have identified and isolated stem cells with the ability to regenerate these specific bones in mice. This breakthrough offers hope for treating conditions like craniosynostosis, a congenital skull deformity that impacts brain development.

Insights from Bone Formation and Regeneration Studies
The study focused on the role of the Axin2 gene in bone formation, crucial for understanding how these stem cells contribute to bone repair. The research also explored mutations linked to craniosynostosis in mice, revealing distinct populations of stem cells unique to skull bones.
Potential Applications in Bone Disease Detection
Beyond repair, identifying these specialized stem cells could aid in diagnosing bone diseases associated with stem cell abnormalities, offering new insights for medical professionals.
Publication and Impact
Published in Nature Communications on February 1, this research marks a significant advancement in bone regeneration therapies, paving the way for future clinical applications and further studies in human subjects.
- Published in Corporate News / Blog
German Stem Cell Scientists Develop 3-D “Mini-retinas” –New Hope for Restoring Sight in Patients with Retinal Degeneration Caused by Diabetes and Inherited Disorders.
Breakthrough in Retinal Regeneration Using 3D Organoids
Protocol for 3D Mini-retinas
German researchers have achieved a significant breakthrough in stem cell technology focused on restoring sight through the development of 3D retina organoids. Published in Stem Cell Reports in March, this study leverages the self-organizing properties of stem cells to create complex, multi-cellular tissue structures.
The innovative protocol involves dividing organoids grown from stem cells into three half-moon shaped pieces during early eye development. This technique facilitates the growth of fully functional retinal cells within each segment, including cone photoreceptors crucial for high acuity and color vision. These advancements are particularly promising for patients suffering from retinal degenerative disorders caused by diabetes and inherited conditions.
Advantages of 3D Retinal Organoids
The development process not only enhances the production yield of retinal organoids by up to four times compared to previous methods but also allows for the formation of more realistic tissue structures resembling natural retinal tissue during development.
Applications in Retinal Disease Research
According to senior author Mike Karl from the German Center for Neurodegenerative Diseases (DZNE) and the Center for Regenerative Therapies (CRTD) at Technische Universität Dresden, the versatility of 3D mini-retinas extends beyond replication of retinal tissue. It offers diverse opportunities for studying retinal diseases and potential therapeutic interventions.
Future Directions in Organoid Research
Karl’s team aims to enhance the complexity of mini-retinas by incorporating blood vessels and studying the regeneration capabilities and neural cell functions specific to the human retina. This approach not only furthers understanding of retinal diseases but also holds promise for developing personalized treatments.
Insights and Comparative Studies
Comparative studies between human pluripotent stem cell-derived retina organoids and in vivo mouse retina underscore the potential of this novel organoid protocol to model retinal diseases effectively.
- Published in Corporate News / Blog
Stem Cell Treatments Normally Used for Cancer Patients are Helping Multiple Sclerosis Patients
Overview of Stem Cell Transplant for MS Patients
Recent reports from the British Broadcasting Corporation (BBC) highlight the transformative effects of stem cell transplant treatments originally used for cancer patients on individuals suffering from Multiple Sclerosis (MS) in the UK. According to a January 18, 2016 report, this innovative approach has shown promising results in restoring mobility and reversing disability in MS patients.
Autologous Hematopoietic Stem Cell Transplantation (HSCT)
The treatment, known as autologous hematopoietic stem cell transplantation (HSCT), involves the infusion of the patient’s own stem cells harvested from bone marrow. This procedure aims to rebuild the immune system, potentially resetting it to a state before it caused MS-related damage.
Testimonials and Clinical Results
Professors Basil Sharrack and John Snowden from Sheffield’s Royal Hallamshire Hospital emphasize the profound impact of HSCT on MS patients. Patients like Steven Storey, who experienced significant disability progression prior to treatment, have reported remarkable improvements in mobility and quality of life post-transplant.
Steven Storey’s Journey
Steven Storey, diagnosed with MS in 2013, transitioned from being an athlete to wheelchair-bound within a year. Following HSCT, he regained movement in his toes within days and achieved unaided standing after four months. While still using a wheelchair, Storey’s progress is described as astounding, allowing him to swim, cycle, and aspire to walk again.
The MIST International Clinical Trial
The Royal Hallamshire Hospital, alongside institutions in the US, Sweden, and Brazil, participates in the MIST trial. This international study evaluates the long-term benefits of HSCT on patients with relapsing-remitting MS (RRMS), aiming to establish its efficacy as a standard treatment option.
Future Prospects and Research
Prof Richard Burt of Northwestern University leads the MIST trial, pioneering HSCT for MS treatment since 1995. Despite challenges from the pharmaceutical and academic sectors, early studies have shown promising neurological disability reductions, with ongoing research poised to validate these findings further.
Conclusion and Outlook
As ongoing research continues to validate the potential of HSCT in treating MS, stakeholders like Emma Gray, M.D., head of clinical trials at the UK’s MS Society, emphasize its life-changing impacts highlighted by real patient experiences. The outcomes from the MIST trial, expected in 2018, could potentially integrate HSCT into the standard healthcare protocols for MS patients in the UK.
- Published in Corporate News / Blog
Global Stem Cells Group Plans Bone Marrow Clinical Trials for Knee Osteoarthritis
Global Stem Cells Group has announced plans to hold clinical trials, pending IRB approval, for bone marrow stem cell treatments targeting knee osteoarthritis. The trials will be held in five GSCG facilities in the U.S. and South America, with 25 patients accepted for each location.
MIAMI, March 31, 2016—Pending Institutional Review Board (IRB) approval, Global Stem Cells Group, Inc. has announced plans to conduct a multi-center, placebo controlled clinical trial to measure the safety and effectiveness of the intra-articular application of freshly isolated bone marrow stem cells for the treatment of osteoarthritis.
The clinical trials, which will begin July 1, 2016 and run for one year, will be held in Global Stem Cell Group facilities in Buenos Aires, Argentina; Bogota, Colombia; Quito, Ecuador; Miami, Florida and Topeka, Kansas. Each center will accept 25 patients per clinical trial, and patients will receive a bone marrow stem cell injection in one knee and a placebo in the other knee..
 The trials are designed to investigate the possible beneficial effect of freshly harvested bone marrow stem cell applications on knee osteoarthritis patients in the control group. Patients will receive standard treatment of bone marrow stem cells intravenously, and will be monitored and assessed for any changes in clinical condition.
The trials are designed to investigate the possible beneficial effect of freshly harvested bone marrow stem cell applications on knee osteoarthritis patients in the control group. Patients will receive standard treatment of bone marrow stem cells intravenously, and will be monitored and assessed for any changes in clinical condition.
Knee osteoarthritis is a chronic, progressive condition affecting an increasing number of people, especially the elderly and obese. It is characterized by degeneration of the cartilage—the natural cushioning between joints inside the knee.
The condition is the result of the wearing away of cartilage. When this happens, the bones of the joints rub more closely against one another with less of the shock-absorbing benefits of cartilage, resulting in pain, swelling, stiffness and a decreased ability to move.
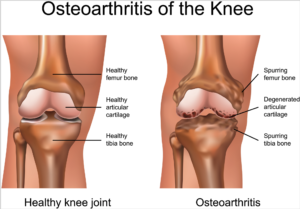 According to the Centers for Disease Control (CDC), knee osteoarthritis will affect 67 million people in the United States by 2030. While conventional treatments like physiotherapy or drugs offer temporary relief of clinical symptoms, total knee replacement is the closest treatment available for permanent relief, which requires invasive surgery, comes at a high cost and is not always successful. The latest advances in stem cell therapies for knee osteoarthritis are designed to restore cartilage function in the knee.
According to the Centers for Disease Control (CDC), knee osteoarthritis will affect 67 million people in the United States by 2030. While conventional treatments like physiotherapy or drugs offer temporary relief of clinical symptoms, total knee replacement is the closest treatment available for permanent relief, which requires invasive surgery, comes at a high cost and is not always successful. The latest advances in stem cell therapies for knee osteoarthritis are designed to restore cartilage function in the knee.
Global Stem Cells Group offers the most advanced protocols and techniques in cellular medicine from around the world.
Details of the protocol and eligibility criteria will be released upon IRB approval.
For more information on Global Stems Cell Group, visit the Global Stem Cells Group website, email bnovas(at)stemcellsgroup(dot)com, or call +1 305 560 5337.
About Global Stem Cells Group:
Global Stem Cells Group, Inc, is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
To view this press release live online, click here
###
- Published in Press Releases
Global Stem Cells Group Announces Manufacturing Phase of Progenikine™ SVF Closed System
Global Stem Cells Group has begun the manufacturing phase of Progenikine™, a new SVF closed system kit utilizing EmCyte technology, containing all the elements necessary to process adipose tissue and obtain stromal vascular fraction in a sterile environment.
MIAMI, March 31, 2016—Global Stem Cells Group, Inc. has announced that Progenikine™, its new and approved SVF closed system kit using EmCyte technology, is in the manufacturing phase and will be available to physicians in July 2016. The Progenikine kit contains all the elements necessary to process adipose tissue and obtain stromal vascular fraction (SVF) in a closed environment.
Adipose derived stem cells (ASCs) are used by physicians for a variety of indications. Most commonly, ASCs are  isolated at the point of care from lipoaspirate (derived from liposuction) tissue as the stromal vascular fraction (SVF), harvested from the patient and immediately administered to the patient as an injection, or used to enrich fat grafts. Isolation of ASCs from adipose tissue is a relatively simple process performed routinely in cell biology laboratories, but isolation at the point of care for immediate clinical administration requires special methodology to prevent contamination, ensure integrity of the clinical procedure, and comply with regulatory requirements.
isolated at the point of care from lipoaspirate (derived from liposuction) tissue as the stromal vascular fraction (SVF), harvested from the patient and immediately administered to the patient as an injection, or used to enrich fat grafts. Isolation of ASCs from adipose tissue is a relatively simple process performed routinely in cell biology laboratories, but isolation at the point of care for immediate clinical administration requires special methodology to prevent contamination, ensure integrity of the clinical procedure, and comply with regulatory requirements.
Developed in conjunction with Patrick Pennie, Emcyte CEO, and and Maritza Novas Director of Research and Development for Global Stem Cells Group, Progenikine  fuses elements from Emcyte systems with the Global Stem cells Group SVF protocols.The kit can provide a low cost, rapid and simple alternative to traditional methods of isolating ASCs, particularly when smaller quantities are needed.
fuses elements from Emcyte systems with the Global Stem cells Group SVF protocols.The kit can provide a low cost, rapid and simple alternative to traditional methods of isolating ASCs, particularly when smaller quantities are needed.
“The Progenikine kit is the newest product designed to help Global Stem Cells Group’s mission to provide accessible products to our member clients, ensuring that more patients will be able to gain access to stem cell therapies,” says Benito Novas, GSCG CEO.
For more information on Global Stems Cell Group, visit the Global Stem Cells Group website,email bnovas(at)stemcellsgroup(dot)com, or call +1 305 560 5337.
About Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About Emcyte:
Fort Myers, Florida-based EmCyte Corporation is a leader in autologous cellular biologics with the GenesisCS Component Concentrating Systems. These systems provide patients with the best opportunity for rapid recovery and provide practitioners with the most advanced clinical point of care experience. EmCyte systems are developed to meet every clinical requirement, giving the physician better clinical choices. EmCyte devices have been independently reviewed and show to produce buffycoat concentrations of 6x to greater than 10x baseline in 7mLs, with yields ranging from 70 percent to greater than 90 percent
EmCyte technology allows for the safe extraction of concentrated platelets and other regenerative cell types from the patient’s own blood. These cells are then re-suspended in a small volume of the patient’s blood plasma and then applied to the treatment site.
###
To view this press release live online, click here
- Published in Press Releases
Our Friend MSCs (Mesenchymal Stem Cells)—Bringing New Life to Old Bones
Understanding MSCs and Their Role in Osteoporosis Treatment
Researchers from the University of Toronto and The Ottawa Hospital have explored the potential of mesenchymal stem cells (MSCs) in treating osteoporosis. MSCs are versatile stromal cells capable of differentiating into bone cells (osteoblasts), cartilage cells (chondrocytes), muscle cells (myocytes), and fat cells (adipocytes).
Research Insights: MSCs Reverse Osteoporosis in Mice
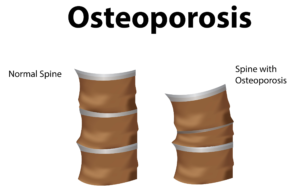
In a groundbreaking study published in Stem Cells Translational Medicine, researchers injected healthy MSCs into mice with osteoporosis. Over six months—a significant period in a mouse’s lifespan—the damaged, brittle bones of the mice were replaced with healthy bone tissue. This restoration underscores the regenerative potential of MSCs in treating bone disorders.
Clinical Applications and Future Directions
This discovery raises hopes for new osteoporosis treatments in humans. Early trials involving elderly patients in the US have shown promising results, with ongoing studies assessing improvements in bone health through biological markers in blood samples.
The Global Impact of Osteoporosis
Globally, over 200 million people suffer from postmenopausal or age-related osteoporosis, leading to approximately 8.9 million bone fractures annually. Current treatments, like Teriparatide, offer temporary relief, highlighting the urgent need for more effective, long-term solutions.
Key Researchers and Publications
Dr. William Stanford, senior scientist at The Ottawa Hospital Research Institute and professor at the University of Ottawa, led the study linking MSC defects to age-related osteoporosis in mice. Co-author Dr. John E. Davies, professor at the University of Toronto, contributed to the study’s publication in Stem Cells Translational Medicine on March 17, 2016.
- Published in Corporate News / Blog