How To Effectively Treat Amyotrophic Lateral Sclerosis (ALS)
What is ALS?
Although a rare neurologic condition, Amyotrophic Lateral Sclerosis (ALS) is the most common type of Motor Neuron Disease (MND), a condition that affects the voluntary muscles. This is a progressive disorder that leads to muscle weakness and depletion due to nerve dysfunction.
ALS is also called Lou Gehrig’s disease, named after the football player who had this condition. The literal meaning of Amyotrophic is ‘no muscle nourishment,’ which becomes the cause of muscle atrophy. ‘Lateral’ refers to the group of nerves in the spinal cord that sends signals to the muscles. It is these nerves that degenerate, leading to sclerosis in this region. In later stages, this affects the nerves that control breathing and hence can be fatal.
Initial Symptoms of ALS
The initial symptoms of ALS include stiffness and muscle weakness, which gradually involve all the muscles under voluntary control. The affected regions and progressive pattern vary from one person to another. Some have difficulty holding a pen or a cup, while others find difficulty speaking, chewing, or even talking. Thus, ALS is an ailment that affects daily life and makes simple tasks painful and troublesome.
ALS Statistics
According to the Center for Disease Control and Prevention (CDC), 14,500 to 15,000 people had ALS in the United States in 2016, with approximately 5000 people having a confirmed diagnosis for the condition annually. Although the average survival rate is three to five years, patients can live for ten years or more.
Types of ALS
Sporadic ALS
This is the most common type and affects 95% of sufferers. This type occurs without a clear cause.
Familial ALS (FALS)
This type occurs in 5-10% of sufferers. This type of ALS is genetic and runs in families. This occurs due to abnormal changes to a gene that is then passed in generations.
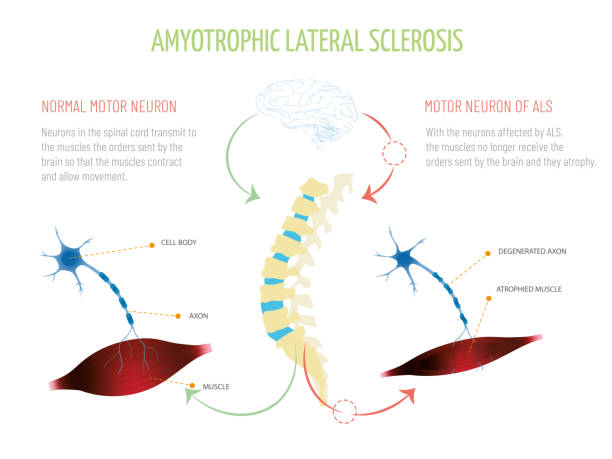
Symptoms of ALS
What are the symptoms of ALS?
Early signs and symptoms might be unnoticeable and become perceptible after some time. Most clinical signs are evident of upper motor neuron and lower motor neuron lesions. The limb onset ALS (70%) involves initial symptoms in the limbs, while the bulbar onset ALS (25%) is characterized by speech and swallowing problems, followed by weakness in the limbs later. The remaining 5% of patients have respiratory involvement in the early period.
Most Common Symptoms Include:
- Muscle weakness in the limbs (distal or proximal)
- Asymmetric progressive muscle wasting
- Difficulty in motor activities like walking, talking, chewing
- Weakness in arms, legs, hands, and feet
- Muscle cramps and twitching
- Slurred speech
- Fatigue
- Emotional liability (episodes of uncontrolled laughing and crying)
- Difficulty in maintaining posture and gait
- Difficulty in breathing and swallowing
With the progression of the disease, symptoms may spread to all parts of the body. In some patients, frontotemporal dementia may occur, resulting in poor memory and decision-making abilities.
Causes of ALS
The exact cause of ALS has not been known by scientists to date. However, research is being carried out to understand what causes ALS. There are several different factors such as:
Genetic Changes
Studies have shown that 5 to 10% of cases of ALS are caused by genetic mutations. For example, changes to the gene that makes SOD1 protein causes damage to motor neurons.
Environmental Factors
No major association has been established between environmental factors like toxins, viruses, diet, or physical trauma and the risk of development of ALS. However, there is ongoing research on the subject. Studies have shown that some athletes are at a higher risk of acquiring ALS due to vigorous physical activity.
Chemical Disturbance
Glutamate is the neurotransmitter that is in control of signals to and from the brain. Accumulation of this neurotransmitter within the spaces surrounding the nerves damages them. Research has also shown mitochondrial structural and functional abnormalities, as well as defects in axonal structure and transport, could be the causative agents for ALS.
Diagnosis of ALS
When it comes to diagnosis, there are no specific tests that can provide a definitive diagnosis for ALS. However, doctors conduct a series of tests to rule other similar diseases. A full medical history check and a neurologic examination are undertaken at regular intervals to assess the progressive worsening of symptoms.
Diagnostic Tests Include:
- Electromyography (EMG): EMG records the electrical activity of the muscle fibers.
- Nerve Conduction Study (NCS): NCS assesses the electrical activity of the nerves and muscles.
- Magnetic Resonance Imaging (MRI): MRI rules out other possible conditions such as a tumor or cyst in the spinal cord, cervical spondylosis, or a hernia in the neck that could be causing the nerve compression.
Laboratory tests such as blood screening and urine tests can also be carried out so that other diseases can be eliminated.
Treatment Options & Management Strategies for ALS
ALS is managed through a multidisciplinary approach. Unfortunately, there is no definitive cure for the disease at this time. Management of ALS is done through symptomatic treatment to ease the condition of the patients and prevent unnecessary complications:
Support
Physicians, psychologists, speech therapists, nutritionists, and home care assistance all play a vital role in making life easier for patients with ALS.
Medication
Riluzole (Rilutek) and Edaravone (Radicava) are the drugs approved by the U.S Food and Drug Administration (FDA) for treating ALS. Riluzole is believed to reduce glutamate levels, thereby decreasing damage to the motor neurons. Edaravone acts as an antioxidant and is believed to expel free radicals and reduce oxidative stress in the motor neurons.
Lifestyle Habits
Physiotherapists can recommend exercise and physical activity like walking, swimming, and bicycling that may improve muscle strength and help elevate mood without overstressing the muscles.
Speech Therapy
Therapists can help patients with ALS to employ strategies to speak clearly. They may also recommend computerized aids such as speech synthesizers and eye-tracking technology to help people learn ways for responding by nonverbal means.
Diet
Nutritionists may formulate a diet plan for patients, which consists of food that is easy to swallow and provides enough nourishment and calories for the patients to maintain adequate energy levels and to prevent excessive weight loss.
Breathing Support
Patients with ALS may suffer from shortness of breath and difficulty breathing during physical activity or while lying down. If this is the case, doctors can recommend Non-Invasive Ventilation (NIV) that provides breathing support through the nose or mouth. NIV improves quality of life and increases the number of years of survival for patients.
Is Stem Cell Therapy an Option?
As previously mentioned, there is no curable treatment for ALS available. However, scientists are researching Stem Cell Therapy as the new favorable approach in the treatment of neurologic disorders. There is a rising interest in Stem Cell Therapy as a promising remedy for curing ALS. Mesenchymal stem cells are particularly believed to be the most suitable ones due to their availability, absence of ethical issues, and positive results in various experiments.
Research on Stem Cell Therapy
Studies and clinical trials have begun to apprehend the benefits of MSC transplantation. They demonstrate that MSCs lead to a partial recovery of motor neurons and a delay in disease progression. Also, there has been no evidence of a major adverse effect after MSC transplantation. When testing this newfound research on animals, the lifespan of the subjected animal has increased with MSC transplantation. These positive results have encouraged the administration of MSC in ALS patients.
However, despite the safe outcomes of MSC transplantation in humans, results show that there is only a partial improvement in ALS sufferers with only a few cases that showed a delay in disease progression. Hence, there is a need for further studies and trials on a higher number of human subjects for a better understanding of MSC effects so that more significant conclusions can be reached.
- Published in Corporate News / Blog
Adimarket, LLC Looks to Expand Market Reach with Launch of New PRP Product
The group’s new platelet-rich plasma (PRP) offering meets international standards for compliance, safety, and efficacy
MIAMI LAKES, Florida—Adimarket, LLC, a subsidiary of the Global Stem Cells Group, today announced the availability of a new product that is a viable treatment option in a host of regenerative medicine sectors. With its new platelet-rich plasma (PRP) product, Adimarket will provide physicians with an exciting treatment protocol that can deliver relief to patients needing critical aesthetic, wound, and orthopedic care.
PRP is a super biological product of sorts, composed of a higher concentration of platelets than what is usually found in blood — 6-10 times more, on average. Adimarket’s PRP kit is the first of its kind in the stem cells and regenerative medicine industry, seeking to deliver higher platelet counts in an affordable and easy-to-use system. The revolutionary system is designed to shorten total processing time and reduce human error as it maximizes platelet concentration numbers. Adimarket’s offering has the capability of producing both leukocyte-rich and leukocyte-poor PRP and works in a variety of centrifuge systems.
PRP has been gaining interest and traction in the world of stem cell therapies and regenerative medicine. For clinicians working in the aesthetics, wound care, and orthopedic sectors, PRP has proven be a promising treatment option in offering relief to their patients.
In developing its PRP product, Adimarket researchers and scientists adhered to the highest international standards, making the product a viable option for physicians across the globe. With the launch of this new product, Adimarket is looking to expand its reach into new markets across the Middle East and Europe.
“Adimarket and its products have been met with great enthusiasm from physicians in the US and Latin America,” said Benito Novas, CEO of the Global Stem Cells Group. “With the launch of this new PRP product, our goal is to continue to serve our partners in those markets while expanding our presence into Europe and the Middle East. Our ultimate goal is to help more physicians across the globe deliver critical stem cell treatments to their patients, with the goal of alleviating unnecessary suffering.”
To learn more about Adimarket and to purchase its PRP product, visit https://www.adimarket.net/. To learn more about the Global Stem Cells Group, visit http://www.stemcellsgroup.com/.
.
- Published in Press Releases
Benito Novas of Aesthetica Marketing Group Set as Keynote for Chilean Aesthetics Congress
The keynote will highlight trends in social media marketing for physicians working in aesthetics
MIAMI LAKES, Florida—Benito Novas, CEO of Aesthetica Marketing Group (AMG), has been selected to serve as the keynote speaker at the upcoming Congreso del la Sociedad Chilena de Medicina y Estética. AMG, which is sponsoring the Chilean congress, is an American company focused on providing doctors working in the aesthetics medical industry practical solutions for their marketing challenges. The congress will be held on August 22-24, 2019 at the Hotel InterContinental Santiago in Santiago, Chile.
The annual event is the group’s main event, attracting physicians in the aesthetics medical industry across Chile and beyond. Novas’ keynote lecture will highlight current trends and best practices in social media and how physicians working in the aesthetics industry can better leverage social media in increasing the success of their practices.
During the congress, AMG will also launch two new courses focused on the aesthetics market, Pellet Therapy Certification and an IV/Chelation course.
The Pellet Therapy Certification course will provide practitioners with comprehensive clinical knowledge of this therapy option—one that is gaining rapid traction and recognition globally. Facilitators will present scientific reviews, case studies, and procedural training during the workshop. Attendees will be provided an in-depth introduction to pellet therapy, supported by reviews of clinical evidence on the need for customized dosing, in addition to assessing pellet dosing protocols for male and female patients. Pellet sterility, equipment, and what to look for in a compounding pharmacy will also be discussed.
AMG’s IV/Chelation course has been redesigned and now includes the most recent practices and protocols in intravenous nutritional therapies, in addition to chelation therapy in regard to its uses in lead toxicity and other heavy metals. Lecturers will not only cover the fundamentals of IV therapy and expand on the use of vitamins, minerals, amino acids, and other unique parenteral compounds but also review various aspects of integrating IV therapy into practice, as well as implementation strategies. Faculty presenters additionally will discuss potential application of the therapy in cancer treatment protocols, thus giving attendees the unique opportunity to learn about the most cutting-edge uses of IV therapy as a treatment method. At the conclusion of the course, clinicians will have a thorough understanding of how to perform IV therapy in both theory and practice.
To learn more about the upcoming Congreso del la Sociedad Chilena de Medicina y Estética or to register, visit https://sochimce.cl/congreso/. To learn more about Aesthetica Marketing Group, visit https://esteticamarketing.com/.
.
- Published in Press Releases
Adimarket, LLC Launches New Product for Physicians Seeking Regenerative Medicine and Stem Cell Centers
The comprehensive guide, “Proposal for Setting Up a Regenerative Medicine and Stem Cell Treatment Center” is available for purchase on Adimarket’s online store.
MIAMI LAKES, Florida—Adimarket, LLC, a subsidiary of the Global Stem Cells Group, has launched a new product for physicians interested in starting regenerative medicine and stem cell treatment centers. The guide, entitled “Proposal for Setting Up a Regenerative Medicine and Stem Cell Treatment Center” provides physicians with valuable information to guide them through the process of proposing and setting up new centers in the industry.
The comprehensive guide provides physicians with a one-stop place to find information critical to setting up a treatment center and includes information about training, equipment installation, cellular product storage procedures, supply inventories, laboratory startup, and more. The guide covers both scientific and administrative training concepts to help enable physicians to start their own centers at the lowest cost possible.
By following the guide, clinic officials will be able to launch a center with a wide range of cell lineages and products available, as well as have at its disposal the industry’s leading patient treatment protocols and standard operating procedures used by the Global Stem Cells Group’s global clinical network.
“For physicians looking to add regenerative medicine protocols to their current practices, Adimarket’s guide is a must-have resource,” said Benito Novas, CEO of the Global Stem Cells Group. “Our hope is that our guide will give more physicians the knowledge, tools, and confidence necessary to offer regenerative medicine and stem cell treatments to their patients to help alleviate suffering.”
To learn more about Adimarket and to purchase the “Proposal for Setting Up a Regenerative Medicine and Stem Cell Treatment Center,” visit https://www.adimarket.net/. To learn more about the Global Stem Cells Group, visit http://www.stemcellsgroup.com/.
.
- Published in Press Releases
Benito Novas to Serve as Keynote Speaker at Mexican Aesthetic Gynecology Congress
The Global Stem Cells CEO will give two additional lectures on marketing and future outlooks for the stem cells industry as well as lead a magistral class for attendees
MIAMI LAKES, Florida—Benito Novas has been invited as a keynote speaker at an upcoming medical congress hosted by the Mexican Board of Aesthetic Gynecology. The group’s fourth congress, the 4to Congreso Internacional de Ginecología Lasar y Funcional, will be held on September 25-28, 2019 at the Holiday Inn hotel in Boca del Rio in Veracruz, Mexico.
Novas, is the Vice President of Public Relations of the International Society for Stem Cell Application (ISSCA), a multi-disciplinary community of scientists and physicians collaborating to treat diseases and lessen human suffering through science, technology, and regenerative medicine. In addition to his keynote speech, Novas will also lecture on two additional topics during the congress as well as lead a magistral class for conference attendees.
Benito Novas is a highly sought-after speaker in the world of stem cell advancements, having served as a successful business owner in the industry with his holding the Global Stem Cells Group. Thanks to Novas’ and his team’s efforts, access to regenerative medicine has expanded exponentially across the globe. Additionally, Novas is a well-regarded marketing professional who has created a successful marketing model for private medical organizations to expand and grow their industry recognition.
Congress attendees will have the opportunity to hear Novas’ talks on the current and future state of stem cells as well as the importance of digital marketing in the medical field. In his lecture on current and future outlooks for stem cells advancement, Novas will share information about current stem cells clinical trials and growing expectations in the industry as well as how FDA policies can influence markets not only in the US but also beyond.
In his lecture on digital marketing, Novas will address why doctors must utilize digital marketing strategies to grow a successful practice. He will look at current trends in how people meet each other and network in today’s increasingly digital world and how social medial plays a role in today’s networking. He will additionally provide strategic tips to attendees on how to capitalize on social media trends to grow practice influence. Some of the tools Novas will discuss include Google Ads and Facebook Ads, as well as what type of content to post to generate engagement with prospective customers and clients.
Novas will also conduct a magistral class following the congress for professionals interested in learning more about digital marketing. During the course, Novas will discuss how practitioners can implement the latest marketing techniques into their practices, including how to create a powerhouse digital marketing team by using platforms such as Fiverr, Upwork, and others.
To learn more about ISSCA’s Miami symposium or to register for the event, visit https://www.issca.us/.
.
- Published in Press Releases
Stem Cells Group to Host Regenerative Medicine Symposium at the University of Miami
ISSCA will partner with SIISDET at the October 2019 event, which will share valuable information with physicians regarding recent breakthroughs in regenerative medicine
MIAMI LAKES, Florida—The International Society for Stem Cells Application (ISSCA), a multi-disciplinary community of scientists and physicians collaborating to treat diseases and lessen human suffering through science, technology, and regenerative medicine, will co-host a symposium in October 2019 with the Sociedad Internacional en Investigación, Salud, Desarrollo Empresarial (SIISDET). The event will be held at the University of Miami on October 22-24, 2019 in the West Ballroom of the Shalala Student Complex.
The Miami symposium will feature educational sessions and product information and demonstrations aimed at doctors looking to incorporate regenerative medicine into their practices. Industry experts will be on hand to share the latest information on the newest compounds in the allogeneic stem cells market, including exosomes, amniotic fluid compounds, and cord blood products.
Allogeneic compounds have gained growing popularity in regenerative medicine due to their increased safety and treatment efficacy. With this in mind, ISSCA representatives will be on hand to give physicians the opportunity to learn more about these popular treatment solutions and how they can be utilized in their practices.
“We are pleased to be able to partner with SIISDET to be able to offer this exciting symposium at the University of Miami,” said Benito Novas, CEO of ISSCA. “We are continually seeking ways to bring the latest advances in regenerative medicine to physicians across the globe. Our Miami symposium will play a critical role in accomplishing this by helping physicians expand their knowledge bases and explore new treatment options in regenerative medicine.”
In addition to educational sessions, the Miami symposium will provide networking and social opportunities for those in attendance. Events on the agenda include an optional boat trip around Miami on the Miami River and a Columbian Day celebration offering live music and Colombian cuisine to symposium attendees.
To learn more about ISSCA’s Miami symposium or to register for the event, visit https://www.issca.us/.
.
- Published in Press Releases
ISSCA Reaches Agreement with Belgium’s Churchill Aesthetic Center
The innovative new center will provide training in regenerative medicine for doctors located in Belgium and France.
MIAMI LAKES, Florida—The International Society for Stem Cell Application (ISSCA), a multi-disciplinary community of scientists and physicians collaborating to treat diseases and lessen human suffering through science, technology, and regenerative medicine, has signed an agreement with Belgium’s Churchill Aesthetic Center. Beginning in July 2019, the center will offer stem cells certification programming for physicians in Belgium and France looking to add regenerative medicine treatment options to their practices.
ISSCA’s Belgian partnership builds on the group’s mission to reach new markets and expand regenerative medicine treatment options to physicians across the globe who want to offer new treatment options to their patients suffering from degenerative diseases and sports injuries. In its most recent clinic openings and partnerships, ISSCA has primarily focused on expanding efforts in the Middle East, US, and Latin America. The group recently set its sights on European expansion, with the partnership in Belgium serving as its second center in Europe, with the other located in Barcelona.
The cutting-edge facility will be equipped by Adimarket, LLC, a subsidiary of the Global Stem Cells Group. Adimarket will outfit the center with equipment and supplies designed to not only teach doctors about the latest protocols in regenerative medicine but also to treat patients suffering from degenerative diseases and sports injuries. Physicians training at the facility will have access to the proven expertise of ISSCA’s global network and training protocols.
“We look forward to ISSCA’s exciting new partnership with Belgium’s Churchill Aesthetic Center and the possibilities it brings in delivering safe and effective regenerative medicine treatment options to more patients across the globe,” said Benito Novas, CEO of the Global Stem Cells Group. “This partnership will help us prepare more physicians to deliver cutting edge treatment options to their patients that help alleviate suffering while also achieving our mission of expanding our global reach.”
To learn more
about Churchill Aesthetic Center, visit https://www.churchill-aesthetic-center.be/.
To learn more about ISSCA,
visit https://issca.us/.
.
- Published in Press Releases
Benito Novas, Global Stem Cells Group CEO Announces Opening of New Stem Cell Center in Valencia, Spain
The new center will continue the Global Stem Cells Group’s mission to expand access to stem cell therapies and training to physicians across the globe.
MIAMI LAKES, Florida—A new Stem Cell Center is set to open its doors in Spain. Benito Novas, CEO of the Global Stem Cells Group (GSCG), a multi-disciplinary community of scientists and physicians collaborating to treat diseases and lessen human suffering through science, technology, and regenerative medicine, has announced the opening of the group’s Valencia, Spain center. The new clinic will be the group’s third operation in Spain and the fourth in Europe.
The Global Stem Cells Group’s Valencia center joins the already existing centers in , which include locations in Bruselas, Madrid, and Barcelona. With the opening of the new center, the GSCG will boast 41 such locations across the globe.
Novas noted that the group has been focusing its efforts on opening its cutting-edge Stem Cell Centers in Europe, in part due to the group’s recognition that regenerative medicine is a fast-growing field across the continent.
“We are pleased to be able to open the doors on a new Stem Cells Center in Europe,” noted Novas. “The GSCG is continually exploring opportunities to deliver new stem-cell based treatment options to patients across Europe, and the Valencia center gives us the opportunity to do this while meeting our strategic mission to expand our presence in Europe.”
In opening the new center, the GSCG has signed an agreement with Dra. Laura Maria Caicedo, medical director of the Instituto Valenciano MEDEC (https://medec.es/conoce-medec/#equipoMedico). Dr. Caicedo is a specialist on hair loss with an interest in delivering stem cell based treatments to her patients and others in Valencia.
“I am thrilled to lead the efforts at the Stem Cell Center in Valencia,” said Dra. Caicedo. “I am looking forward to utilizing cutting-edge therapy options based in stem cell science to help treat patients suffering from hair loss. Additionally, the opportunity is an exciting one given the Global Stem Cells Group’s excellent reputation and stature as the longest-standing and most respected stem cells network. I’m looking forward to learning from other physicians in the network across the globe and sharing my expertise with other practitioners in regenerative medicine.”
The GSCG will provide the Valencia center with the newest and most effective marketing tools in the field to help the franchise increase its performance in recruiting new patients.
Marketing support is one of the many benefits of joining the GSCG’s expanding global network of clinics. Being part of the group’s network gives physicians access to other experts within the its global network, add to their practice the newest regenerative medicine protocols, and have access to a proven marketing strategy developed by GSCG.
To learn more about the Global Stem Cells Group, visit http://www.stemcellsgroup.com/.
.
- Published in Press Releases
ISSCA Set to Host Regenerative Medicine Symposium in Buenos Aires
The global group will hold its 6th International Regenerative Medicine Symposium in October 2019.
MIAMI LAKES, Florida—The International Society for Stem Cell Application (ISSCA), a multi-disciplinary community of scientists and physicians collaborating to treat diseases and lessen human suffering through science, technology, and regenerative medicine, has announced its 6th annual International Regenerative Medicine Symposium in Buenos Aires. The event will attract physicians from across the globe and will be held on October 12, 2019 in Buenos Aires, Argentina at the Hotel Panamericano.
The past five years of the ISSCA sponsored event have been a global success. This year’s meeting will feature educational sessions and product information and demonstrations, with a special focused placed on the newest compounds in the allogeneic stem cells market, including exosomes, amniotic fluid compounds, and cord blood products. With allogeneic compounds growing in popularity, safety, and treatment efficacy, ISSCA will give physicians the opportunity to learn more about them and how they can be incorporated into their practices.
A number of experts within the field of regenerative medicine are confirmed for the event and include.
• Dra. Maritza Novas, Chief of I&D, Global Stem Cells Group USA
• Dr. Leopoldo Paradas, President of Asociación Chilena de Ingeniería Tisular
“We are pleased to be hosting our popular International Regenerative Medicine Symposium for a sixth year,” said Benito Novas, CEO of ISSCA. “Each year our symposium focuses on critical topics and exciting breakthroughs in the field of regenerative medicine, and this year is no different as we seek to retain our position as a vanguard in the field.”
Physicians interested in registering for or learning more about the Buenos Aires symposium may visit https://www.issca.us/.
.
- Published in Press Releases
ISSCA Launches New Stem Cells Certification Course
The newly developed program gives physicians the opportunity to learn the latest methods and knowledge in the field of regenerative medicine.
MIAMI LAKES, Florida—The International Society for Stem Cells Application (ISSCA), a multi-disciplinary community of scientists and physicians collaborating to treat diseases and lessen human suffering through science, technology, and regenerative medicine, has announced a new course for physicians interested in gaining certification in regenerative medicine. The course was developed out of the group’s highly successful global conference certifications, providing physicians a newly designed and more intimate and personalized smaller group training through which to gain critical skills and knowledge in the field of regenerative medicine.
Through completion of the certification course, physicians will learn how they can add cell-based product protocols to their existing medical practices, including those using exosomes, amniotic fluid, and cord blood.
A host of global experts practicing within the field will be on hand to lead attendees through the new training. They will share first-hand scientific information on the science behind cell therapies and cell-based products and their clinical applications. In-depth examination of clinical trials that demonstrate the safety and efficacy of cell-based products will also be part of the one-day training.
Physicians attending the certification will also learn how to distinguish between the most popular cellular products on the market and what factors to keep in mind when choosing stem cell protocols as a therapeutic alternative. Course faculty will additionally lead attendees through how to structure treatment plans and protocols for patients who may benefit from stem cell therapies.
“We are pleased to be able to offer this exciting and informative new training to physicians interested in adding stem cell treatment options to their current medical practices,” said Benito Novas, CEO of ISSCA. “We seek to remain at the forefront of the stem cells field by continuously innovating and offering the global community the training they need to deliver effective, cutting edge treatment options to their patients. We decided to offer this new training in part due to the demand for it from our customers, who are eager to expand their knowledge base and offer new treatment options to their patients.”
ISSCA has set four dates and locations for its new certification course, and this is the first time such certification has been held by the group outside of the United States:
• Santiago de Chile, Chile: September 5, 2019
• Bogota, Colombia: October 4, 2019
• Buenos Aires, Argentina: October 11, 2019
• Miami, Florida (in English): November 1, 2019
To learn more about ISSCA’s latest certification program and to register, or to learn more about ISSCA, visit https://www.issca.us/.
- Published in Press Releases