Bogota Course Will Share the Latest in Stem Cell Treatment Protocols
The two-day certification program is sponsored by ISSCA, will feature three topics new to the group’s Colombian training circuit
MIAMI LAKES, Florida— The International Society for Stem Cell Application (ISSCA), a global leader in stem cells research, applications, and education, has announced their latest certification program to be held in Bogota, Colombia on March 13-14, 2020.
The Stem Cells Certification course will feature a number of educational and hands-on sessions designed to give physicians the tools needed to integrate regenerative treatments into their practices. Registration for the two-day course is limited to eight participants to allow for intensive training and interaction.
During the two-day training, ISSCA faculty will share the latest stem cell protocols, including how to harvest and process stem cells derived from Stromal Vascular Faction (SVF) tissue and bone marrow.
ISSCA faculty will additionally introduce three new protocols never before covered in past courses held in Colombia. The new curriculum includes clinical applications of allogeneic stem cells; using GCell technology, a device that can process SVF without enzymatic compounds; and an introduction to NK cell protocols, the newest regenerative medicine treatment to fight cancer.
“Our two-day training in Bogota will provide physicians with an invaluable opportunity to receive intensive hands-on training and education in some of today’s most effective and cutting-edge stem cells protocols,” said Benito Novas, ISSCA VP of Public Relations. “Physicians will learn from global leaders in the stem cells industry and be able to take practical application strategies back with them to integrate into their own practices to help patients suffering from degenerative diseases.”To learn more about the ISSCA, visit www.issca.us. More information about the Bogota certification course can be found at http://cursocelulasmadre.com/entrenamiento-bogota-programa/
- Published in Press Releases
Clinical trials on NK cells give hope for many people who are suffering from cancer.
Induced Pluripotent Stem Cells (iPSCs)
Induced pluripotent stem cells (also known as iPS cells or iPSCs) are a type of pluripotent stem cell that can be generated directly from a somatic cell. Pluripotent stem cells hold promise in the field of regenerative medicine. Because they can propagate indefinitely, as well as give rise to every other cell type in the body (such as neurons, heart, pancreatic, and liver cells), they represent a single source of cells that could be used to replace those lost to damage or disease.
Natural Killer Cells and Their Role
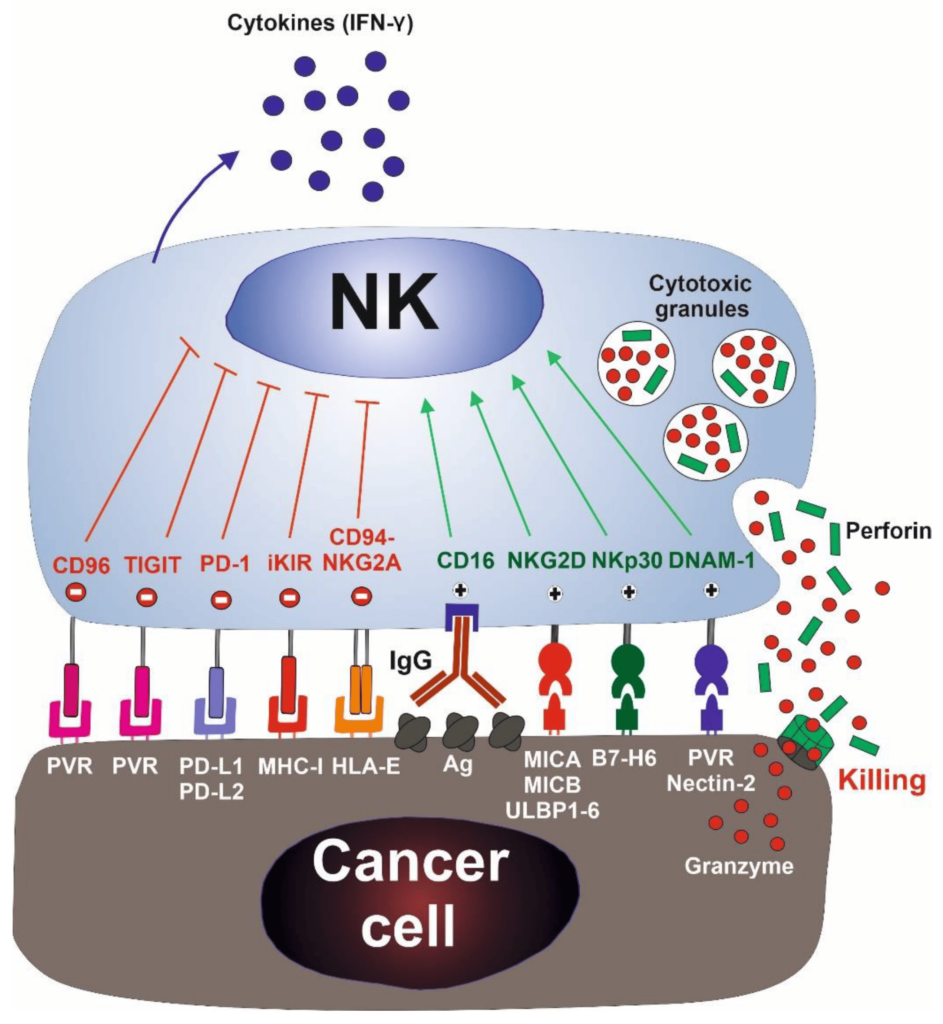
Natural killer (NK) cells are a type of cytotoxic lymphocyte critical to the innate immune system. The role NK cells play is analogous to that of cytotoxic T cells in the vertebrate adaptive immune response. NK cells provide rapid responses to virus-infected cells, acting at around three days after infection, and respond to tumor formation.
Typically, immune cells detect the major histocompatibility complex (MHC) presented on infected cell surfaces, triggering cytokine release and causing apoptosis. NK cells are unique, however, as they can recognize stressed cells in the absence of antibodies and MHC, allowing for a much faster immune reaction.
Clinical Trials on NK Cells
Overview of NK Cell Clinical Trials
In a first clinical trial, a natural killer cell immunotherapy derived from induced pluripotent stem cells is being tested for safety in 64 patients with a variety of solid tumors. The first subjects used for the study received the cells in February at the University of California, San Diego (UCSD) Moores Cancer Center and MD Anderson Cancer Center.
Targeted Cancer Types
This study targets late-stage cancer patients with solid tumors, including lymphoma, colorectal cancer, and breast cancer. The FT500 NK cells do not undergo any further alterations after their derivation from induced pluripotent stem cells (iPSCs), offering the possibility of a quicker, ready-made treatment.
Human Embryonic Stem Cells and iPSCs
Human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) provide an accessible, genetically tractable, and homogenous starting cell population to efficiently study human blood cell development. These cell populations provide platforms to develop new cell-based therapies to treat both malignant and nonmalignant hematological diseases.
NK Cells and Cancer Cell Targeting
The NK cells are immune cells in the same family as T and B cells and are very good at targeting cancer cells for destruction. Laboratory experiments have shown they do so by attacking cells that have lost their significant self-recognition signals that tell the immune system not to attack. This is the phenomenon that can happen among cancer cells but not healthy cells. Experts are not sure how many cancer cells lose that signal. Researchers are hopeful that the clinical trial can help determine which cancer patients could benefit the most from NK cell treatment.
iPS Clones and Tumor Formation
The ability to induce pluripotent stem cells from committed, human somatic cells provides tremendous potential for regenerative medicine. However, there is a defined neoplastic potential inherent to such reprogramming that must be understood and may offer a model for critical understanding of events in the formation of tumors. Using genome-wide assays, we identify cancer-related epigenetic abnormalities that arise early during reprogramming and persist in induced pluripotent stem cell (iPS) clones. These include hundreds of abnormal gene silencing events, patterns of aberrant responses to epigenetic-modifying drugs resembling those for cancer cells, and the presence in iPS and partially reprogrammed cells of cancer-specific gene promoter DNA methylation alterations.
Modification of T Cells for Cancer Therapy
Progress in Adoptive T-cell Therapy
Progress in adoptive T-cell therapy for cancer and infectious diseases is hampered by the lack of readily available antigen-specific human T lymphocytes. Pluripotent stem cells could provide an estimable source of T lymphocytes, but the therapeutic potential of human pluripotent stem cell-derived lymphoid cells generated to date remains uncertain.
CAR T-Cell Therapy
Recently, some approved cell therapies for cancer also rely on modifying T cells, in those cases to produce cancer cell–binding chimeric antigen receptors (CARs), and have been effective in treating certain cancers such as leukemia.
Application of CAR T-Cell Therapy in Solid Tumors
The CAR T technology has wowed the field by all but obliterating some patients’ blood cancers, but solid malignancies present new challenges.
Therapies containing such chimeric antigen receptor (CAR) T cells have been approved for some types of so-called liquid cancers of the blood and bone marrow, such as large B-cell lymphoma and B-cell acute lymphoblastic leukemia. However, the approach has not had as much success for solid tumors.
Challenges and Innovations
Serious research into the therapy for brain cancer started almost 20 years ago after cancer biologist Waldemar Debinski, then at Penn State, discovered that the receptor for the immune signaling molecule interleukin 13 (IL-13) was present on glioblastomas, but not on healthy brain tissue. The receptor thus seemed like an excellent target to home in on cancer cells while sparing healthy ones. The CAR spacer domain that spans the immune cells’ membranes and its intracellular co-stimulatory areas, as well as the process used to expand cells outside the body, boosts the T cells’ activity.
CAR T-Cell Therapy: A Safer Approach
While managing CAR T-cell therapy toxicity could help keep already-designed treatments on their march to the clinic, many immunotherapy companies are also working to develop a new generation of inherently safer therapies, yet just as efficient. A crucial part of achieving this goal will be improving CAR specificity for target cells. With current treatments, the destruction of normal cells is often an unavoidable side effect when healthy tissue carries the same antigens as tumors; noncancerous B cells, for example, are usually casualties in CD19-targeted therapies.
Delivery Challenges
CAR T delivery is a challenging factor in the treatment of solid tumors and other unknown forms of tumors. With non-solid cancers, cells are administered by a blood infusion, and once in circulation, the CAR T cells can seek out and destroy the rogue cells. For solid tumors, it’s not so simple.
Future Directions
The main drawback of taking cells from a patient and developing them into a cellular immunotherapy product is that the process can take weeks.
Patel tells The Scientist, “But for the majority of patients who may not be a candidate or may not have time to wait for such an approach, the idea that there’s off-the-shelf immunotherapy that could potentially act as a living drug against their cancer, I think is a fascinating concept.”
- Published in Corporate News / Blog
Stem cell treatment could offer one-end-solution to Diabetes
Insulin-producing cells grown in the lab could provide a possible cure for the age-old disease, diabetes.
Understanding Type 1 Diabetes
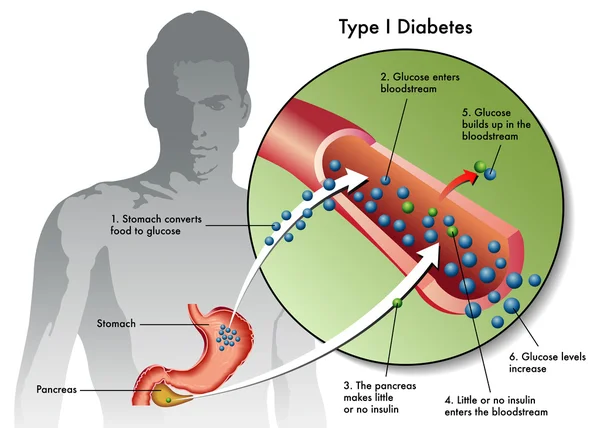
Type 1 diabetes is an autoimmune disease that destroys insulin-producing pancreatic beta cells, leading to dangerously high blood glucose levels. Patients often require insulin administration and other medications to manage blood sugar levels. For those who struggle with blood sugar control, beta-cell transplants are an option, but they require immunosuppressive drugs.
Breakthroughs in Stem Cell Research
A research group at Harvard University reported using insulin-producing cells derived from human embryonic stem cells (ESCs) and induced pluripotent stem cells to lower blood glucose levels in mice. Laboratories worldwide are making rapid progress in human stem cell technology to develop cells functionally equivalent to beta cells and other pancreatic cell types. Novel biomaterials are being developed to encapsulate these cells, protecting them from the immune system without the need for immunosuppressants.
Major Investments in Diabetes Treatment
Pharmaceutical companies and venture capital firms have invested over $100 million in leading biotechnology companies to bring stem cell treatments into clinical use. Prominent companies include:
- Semma Therapeutics
- Sigilon Therapeutics
- ViaCyte
Transforming Human Stem Cells into Insulin-Producing Cells
Researchers at UC San Francisco have successfully transformed human stem cells into mature insulin-producing cells, a significant breakthrough in developing a cure for Type 1 Diabetes (T1D). These cells mimic the function of pancreatic beta cells and offer hope for creating transplantable cells for patients with diabetes.
What is T1 Diabetes?
Type 1 diabetes is characterized by the destruction of insulin-producing beta cells in the pancreas, typically starting in childhood. Without insulin, blood glucose levels can spike, causing severe organ damage and potentially leading to death. The condition can be managed with insulin shots, but serious health complications often persist, such as kidney failure, heart disease, and stroke.
Making Insulin-Producing Cells from Stem Cells
Diabetes can be treated through a pancreas transplant or donor cell transplantation, but both methods rely on deceased donors. Scientists have struggled to produce mature beta cells responsive to blood glucose and capable of secreting insulin correctly. However, recent breakthroughs at UCSF have achieved this transformation in mice, marking a critical step toward human application.
Protecting Stem Cell Therapies from the Immune System
Delivering stem cell therapies requires protection from immune attacks while maintaining cell functionality. Companies are exploring two main strategies:
- Microencapsulation: Immobilizing cells individually or in small clusters within biocompatible gel blobs.
- Macroencapsulation: Placing larger numbers of cells into a larger, implantable device.
Companies Leading the Way
- ViaCyte: Partnered with Johnson & Johnson, using microencapsulation in clinical trials.
- Semma: Developing both macro- and microencapsulation methods, readying for clinical trials.
- Sigilon: Utilizing gel-based spheres for encapsulation, in collaboration with Eli Lilly.
Conclusion
Stem cell treatment holds the potential to revolutionize diabetes care, offering a one-end solution to managing and potentially curing the disease. With significant investments and ongoing research, the future of diabetes treatment looks promising. Researchers and companies are making strides in developing effective and safe stem cell therapies, bringing hope to millions worldwide.
- Published in Corporate News / Blog
Bats Carry Corona Virus. So Why Don’t They Get Sick?
A lot of viruses that have taken a toll on life, such as the Ebola virus in Africa, the Nipah virus, and the most recent coronavirus that left China running helter-skelter, all seemed to have originated from bats.
Origins of the Coronavirus
During the course of the virus epidemic in Wuhan, where it was first detected, some Chinese researchers in Wuhan examined some patients affected in that area and took samples of the virus. They did findings on the genetic sequence of the virus with other viruses that were known. The coronavirus surprisingly had a 96% match with the horseshoe bats that are dominant in the southwest of China. The research findings were then published in a study in February 2020.
A virologist Vineet Menachery from the University of Texas Medical Branch at Galveston, though not affiliated with the study, said, “They’re too close in terms of their pure genetics to say they’re not related, or that they didn’t have a common ancestor.” Menachery was a reputable virologist and had done other research works. He contributed to the theory that the spread of the coronavirus must have been from these bats to humans and possibly must have had another animal that served as an intermediary for the spread.
Similar Cases of Virus Transmission
This same thing had happened with other forms of coronaviruses as noted in the case of SARS (Severe Acute Respiratory Syndrome), an outbreak that took place in 2002-2003 where civets, a mongoose family member, were infected with the bat coronavirus and spread as humans bought them for food. Another case was the MERS (Middle East Respiratory Syndrome) outbreak. This one happened in 2012 and was a result of infected camels from the virus. People who ate undercooked meat of camels and drank the raw milk of camels were all affected.
Why Don’t Bats Get Sick?
So why is it that there are so many diseases that are spread from bats? It’s no doubt, bats have a lot of viruses that they carry with them. And these viruses, in their variety, are spread and manifest their tolls on people. Scientists are not sure why this is the case as confirmed by Kevin Olival, a research vice president at EcoHealth Alliance, a non-profit organization based in the U.S. He went further to say that it may have something to do with the family of the viruses carried by the bats. There are over 130 different families of viruses that bats do carry around. And then, most bats and humans do come in contact through several means. The millions of populations of bats are ubiquitous to all the continents apart from Antarctica. Rebekah of Colorado State University who researched infectious pathogens said, “There’s a lot of viruses we’re finding in bats because there’s a lot of bats out there.”
Bats and Their Unique Biology
Bats move about in multitudes and live in colonies of large populations. Some of these members live in caves and share caves and trees where there can be contact between humans and bats. Hence, these viruses can spread from these bats to humans. Despite their sizes, bats have relatively long lifespans and can live over 30 years. “So there’s a long time for them to be persistently infected with the virus and shed it into the environment,” Kading says. The modes of mechanisms for these viruses are through urine, saliva, and feces of bats. The outbreak of Nipah that happened in Bangladesh was linked to the sap of a date palm gotten from some trees that some bats licked and had infested with their urine.
Reading through all these, it is not absurd to wonder why the bats themselves do not get affected by the viruses they carry. The answer to that question is based on the fact that the bat is the only flying mammal in the world. Their body metabolism and processes quite differ from those of normal mammals too. When bats fly, their heart rates rise to about a thousand beats per minute with a temperature rise of about 100 degrees Fahrenheit. Linfa Wang, a student of bat viruses at Duke-NUS Medical School in Singapore, says that when these signs manifest in other mammals, they are signals that can trigger death. But this is not the same case for bats. This is a lifestyle for them every day. Their system is also capable of producing molecules that other organisms do not have. The molecules carry out repair functions and prevent cell damage. This makes their system a bit irresistible to infections and also makes them recalcitrant to viruses and resilient to diseases such as diabetes, cancer, and other health conditions. This is proof that the manifestation of viruses in mammals is not always as a result of the virus itself but as a result of the body’s reaction to the presence of such a virus that makes us ill by triggering other chain reactions, as Wang explains.
Human Activities and Virus Transmission
Olival at EcoHealth explains that these bats have coevolved with these viruses and it is not totally their fault that we humans are infected and affected by these viruses. The actual problem is when the viruses move from their species to other species of mammals which is also fostered by human activity. Naturally, it would be hard for most animals and mammals to cross paths. But Olival says that the presence of some activities and availability of exchange platforms made available by humans can allow such interaction to occur. She gave an example using wildlife markets like the one in Wuhan, where a bat could be mixed up with a civet, which later on comes in contact with humans – e.g., butchers who do not observe proper hygiene and protection from animal blood.
“The way that we’re coming into contact with these animals, hunting, selling, and trading them is to a scale that really we haven’t seen before,” he says. Investigative teams did some in-depth search and they discovered some traces of this virus in 22 stalls and in a garbage truck that was found at Huanan Seafood Market right there in Wuhan, a place known for booming trade for live animals. This discovery led to shutting down the market as it was tied to the majority of the cases.
Conclusion
The intermediary animals to these viruses are still a mystery, but it is clear that some of these animals are prone to interact more with humans. This is why when they are infected, the likelihood for human infection is widened. These other infected animals can sneeze, urinate, be cooked as food, or even owned as pets. Bats are not just vectors for viruses, they play an important role in balancing the ecosystem. They feed on insects and fruits and are active agents of pollination. In fact, Wang believes that since these bats have successfully coevolved with these viruses, there is every possibility that they can be the agents that can lead to the cure and provision of therapies for these viruses.
- Published in Corporate News / Blog
Mexico City Medical Congress to Showcase the Global Stem Cells Group’s Latest Innovations
The group is sponsoring the event, which will attract physicians from across the globe with an interest in longevity and anti-aging medicine
MIAMI LAKES, Florida— The Global Stem Cells Group (GSCG) is set to sponsor the XI Congreso Mundial de Medicina Antienvejecimiento y Longevidad (World Conference of Anti-Aging and Longevity Medicine) to be held in Mexico City, Mexico on February 16-18, 2020.
The medical congress is expected to attract over 450 physicians and researchers from across the world interested in anti-aging and longevity practices and medical innovations. Over 30 speakers are slated to share information with attendees on a wide range of topics on how to lead a long, healthy life and improve longevity.
The GSCG is set to share a number of its latest innovations with congress attendees, including its newly released GCell technology device. This cutting-edge tool utilizes micrograft technology to harness the natural and powerful restorative capabilities of adipose tissues. Because it is FDA compliant, the device allows physicians across the globe to continue practicing adult stem cells-based procedures.
Additional benefits of GCell technology include shorter treatment times, delivering in-office treatments in around 30 minutes with local anesthesia, as well as less fat collection compared to existing treatments (15 mL versus 50 mL). GCell technology holds exciting implications across a range of medical specialties, including orthopedics, dermatology, cosmetic gynecology, aesthetics, and hair loss.
In addition to its GCell technology, the GSCG will also feature its newest line of stem cells products derived from first-tissue exosomes. Cellgenic Flow Exosomes utilizes the latest science and research available in cellular therapies to deliver a non-surgical approach to creating regenerative responses in a broad range of treatments. The product utilizes exosomes, which replicate the signals given out by stem cells, versus actual stem cells. Exosomes play a pivotal role in cell-to-cell communication and are involved in a wide range of physiological processes. These particles transfer critical bioactive molecules such as proteins, mRNA, and miRNA between cells and regulate gene expression in recipient cells.
“The XI Congreso Mundial de Medicina Antienvejecimiento y Longevidad is one of the world’s premier events connecting physicians and researchers with today’s most innovative treatments and technologies utilizing regenerative medicine,” said Benito Novas, CEO of the GSCG. “As a worldwide leader in training, education, and innovative products in the field of regenerative medicine, the GSCG is pleased to sponsor this congress and share its exciting new portfolio of products with physicians from across the world.”
To learn more about the Global Stem Cells Group and all of the group’s latest news and innovations, visit http://www.stemcellsgroup.com/
- Published in Press Releases
Two Global Stem Cells Group Officials to Speak at Paraguay Conference
The group is set to sponsor the 2nd Congress of Aesthetic Medicine and Regenerative Gynecology to be held in Paraguay
MIAMI LAKES, Florida—The Global Stem Cells Group (GSCG) has announced its sponsorship of the 2nd Congress of Aesthetic Medicine and Regenerative Gynecology to be held on March 12-13, 2020 in Asuncion, Paraguay. The GSCG’s CEO Benito Novas and Development and Research Director Dra. Maritza Novas will each deliver key lectures during the annual medical congress, which will focus on advancements in the fields of aesthetic medicine and regenerative gynecology.
Benito Novas serves as the GSCG’s CEO and will lead a lecture on regenerative medicine marketing strategies. His talk will give practical marketing tools and advice to physicians running their own private practices in aesthetic medicine. In addition to his role as CEO with the GSCG, Novas also serves as the Head of Public Relations for the International Society for Stem Cell Application (ISSCA) and has authored two books on marketing strategies for clinics in the regenerative medicine industry, Your Aesthetic Practice: What Your Patients Are Saying and Marketing Digital en Su Clínica Estética (https://www.thriftbooks.com/a/benito-novas/2752773/).
Dra. Maritza Novas serves as the GSCG’s Development and Research Director as well as the US Director for the ISSCA. She will deliver the opening lecture for the congress, discussing clinical applications of regenerative medicine in the aesthetic field. Her lecture will be informed by clinical case studies demonstrating the efficacy of regenerative treatments in aesthetics. Dra. Novas is well-renowned in the field of regenerative medicine for making a profound impact on her patients through innovative treatment protocols. She has additionally trained countless doctors looking to add regenerative medicine to their practices.
On March 14, following the conclusion of the congress, representatives from the GSCG will also offer a certification course in cellular therapy. The course is designed to help all physicians who want to add stem cells therapies based on birth tissue derived components to their practices. Attendees will learn about the latest stem cell products, such as exosomes, cord blood, and amniotic fluid. Additional topics to be covered will include how to choose the right products to treat specific conditions and details about how to handle the manufacturing process, from selecting a healthy donor through quality control and storage at the medical office.
“The Global Stem Cells Group is pleased to serve as a sponsor for the 2nd Congress of Aesthetic Medicine and Regenerative Gynecology,” said Benito Novas. “We look forward to sharing today’s best research on marketing strategies to help regenerative medicine practices grow their success and influence as well as sharing clinical applications of stem cells treatments in aesthetic medicine. As a global leader in stem cells education, we always look forward to these events to help more physicians gain the knowledge and skills necessary to bring life-saving treatments to more patients across the globe.”
To learn more about the Global Stem Cells Group and all of the group’s latest news and innovations, visit http://www.stemcellsgroup.com/.
- Published in Press Releases
Global Stem Cells Group to Sponsor Miami Congress on Anti-Aging and Longevity
The group will launch its innovative GCell technology and a new online course at the event, while CEO Benito Novas will lead a lecture about marketing in the regenerative medicine field
MIAMI LAKES, Florida—The Global Stem Cells Group (GSCG) is set to sponsor the Second Intercontinental Medical Congress on Anti-Aging and Longevity (https://miami2020.semal.org/) hosted by the Spanish Society of Anti-Aging Medicine (SEMAL) and the Federation of Latin American of Anti-Aging Medicine (FISMAL). The congress will be held on February 6-9, 2020 in Miami, Florida at the Hampton Inn & Suites by Hilton Miami Brickell.
During the event, the Global Medical Group, a subsidiary of GSCG, will launch its GCell micrograft technology, an autologous tissue suspension that boasts minimal manipulation. The product has application implications for a wide range of medical uses, including orthopedics, dermatology, cosmetic gynecology, aesthetics, and hair loss.
GCell technology will allow physicians utilizing regenerative medicine to continue practicing adult stem cells-based procedures thanks to the product’s adherence to FDA compliance standards since the procedure does not utilize enzymes.
GCell technology is a closed-system medical device that harnesses the natural and powerful restorative capabilities of adipose tissue. The GCell SVF (Stromal Vascular Fraction) procedure can be performed in office in around 30 minutes, while existing procedures can take up to two hours. It also utilizes only local anesthesia, and performing physicians only have to collect 15 ml of fat instead of the 50 ml required for traditional protocols.
During the congress, the GSCG will also be launching its new online stem cells course, which will benefit doctors interested in learning about the newest stem cells protocols and technologies. The course will specifically focus on stem cells protocols based on first tissue derived compounds, such as exosomes, cord blood, and amniotic fluid.
In addition to launching GCell technology and its new course, the GSCG’s CEO and founder Benito Novas will be giving a lecture in the main room. He will be speaking to attendees about the newest challenges facing the regenerative medicine filed and what types of marketing strategies practitioners should implement to run a successful practice integrating regenerative medicine.
“The Global Stem Cells Group is looking forward to attending the Second Intercontinental Medical Congress on Anti-Aging and Longevity to talk with attending physicians and researchers about the technological and educational advances we are making to help move the field of regenerative medicine forward,” said Novas.
To learn more about the Global Stem Cells Group and all of the group’s latest news and innovations, visit http://www.stemcellsgroup.com/.
- Published in Press Releases
A New Medical Device, in the Management of Complex Wounds
Innovative Wound Healing: The Gcells Solution
Because of the complex nature of the wound healing process, an injury on the skin can pose several challenges and likely complications, especially when acute. They can deteriorate from acute to chronic conditions, requiring external intervention best understood by a specialist physician to restore normalcy to the affected area.
The Complexity of Wound Healing
The complexity of wound healing and research remains an ocean of knowledge that is continuously researched intensely to uncover depths of wound healing techniques and interventions. Hence, this report contains an introduction and details on the use of a new medical innovation called Gcells, used primarily for the management of wounds of different etiologies.
Gcells: A Breakthrough in Wound Management
In cases where the process of wound healing seemed difficult, Gcells proved great effects, attributed to their design and working protocol. Gcells are conditioned to work with an enriched suspension of progenitor cells that can efficiently aid the tissue repair process. In this case report, two subjects were used as donors and acceptors of these micro-grafts.
Understanding the Skin’s Repair Mechanism
The skin is the outer layer of the body, offering protection to the underlying layers. A wound breaks this layer, inhibiting its various functions and potentially exposing or breaking the underlying tissue layers. Repair processes are inherent and part of homeostatic processes of the body to try to restore the skin to its normal structure and function.
Basic Wound Healing Process
The basic process for skin repair involves clot formation and inflammation, where vessels dilate and monocytes activate, leading to the breakdown of necrotic tissues. This basic process can be inhibited or delayed by various factors, leading to the transformation of acute wounds to chronic forms. If there is no alteration in the repair process, mesenchymal cells kickstart the proliferative process, beginning to repair and restructure the affected tissues from the base. At the same time, epithelial tissues begin to grow around the wound, leading to the final step of the healing process: skin remodeling and scar maturation.
Factors Affecting Wound Healing
These processes work best under certain conditions. Factors such as cardiovascular ailments, diabetes, bacterial infections, or other types of infections can inhibit these processes.
Importance of Mesenchymal Cells
During the proliferative phase of wound healing, mesenchymal cells are key players. Their structure includes mesenchymal stem cells (MSCs), which are multipotent and offer supportive, therapeutic, and trophic functions. They can release viable trophic, anti-inflammatory cytokines, and anti-apoptotic molecules that offer protection during the repair of wounded skin. MSCs also possess subpopulations with stem-like nature, commonly referred to as “side population” (SP) cells. These cells are enriched in progenitor and multipotent stem cells and exist in various tissues and tumors.
Research on Stem Cells
Research has shown that these stem cells can differentiate into osteoblasts and build a woven bone by forming an extracellular matrix (ECM) secreted by the osteoblasts. This discovery demonstrated the potential of dental pulp as a rich source of progenitor/autologous cells that can aid in healing processes, including the regeneration of craniofacial bones.
Gcells Innovation: Working Principle
Gcells successfully separate this side population with a size of 50 microns. At this cell population, they can form autologous micro-grafts that can be used alone or alongside biomaterials prepared in a biocomplex ready for use when necessary.
Case Report Overview
In this case report, two subjects were used as donors and acceptors of these micro-grafts for enhanced healing of complex wounds through autologous micro-grafts using Gcells.
Clinical Case 1: Abdominoplasty Bariatric Complications
The first case involves a 50-year-old woman with no diseases or disorders. She underwent a laparoscopic gastric bypass surgery and was doing well with weight loss parameters. Two years later, she moved in for abdominoplasty bariatric surgery. Post-surgery complications showed necrosis, discovered after the first medical examination, with tissue loss of about 150 to 200cm² at the end of the flaps.
Initial Treatment
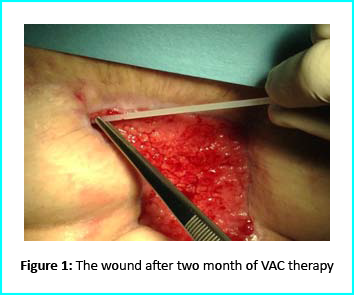
An initial necrosectomy showed an intense loss of tissue, and the wound was placed on VAC therapy. The patient actively participated in this therapy for one week, then continued at home as an outpatient. The VAC therapy showed progressive wound cleansing and granulation tissue formation around the base area.
Gcells Intervention
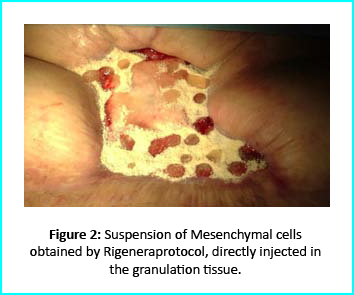
The Gcells protocol started after consent from the outpatient. A 3cm² skin sample was collected from the patient to obtain the cell suspension needed to be injected into the granulation tissue. Conventional wound treatment followed, including cleaning and replacement with sterile gauze dabbed with Vaseline.
Outcome
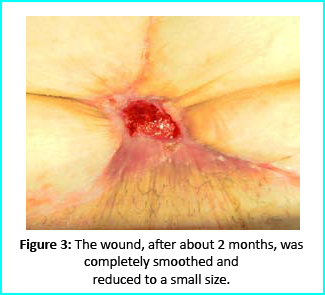
The wound area began to improve in healing progress and general appearance. In two months, the undermined area disappeared and leveled with the skin surface. Two months later, the wound reduced to a very mild and smooth scar compared to the initial condition.
Clinical Case 2: Complications from Liver Cirrhosis and Surgery
The second case involves a 78-year-old man who suffered from liver cirrhosis, hiatal hernia, and diabetes. He underwent complex surgery, including distal esophagectomy and ileostomy protection. Post-surgery complications included necrotic tissues around the biological prosthesis.
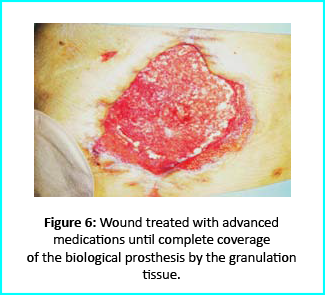
Initial Treatment
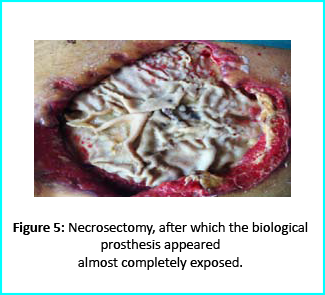
Necrosectomy was conducted, leaving the biological prosthesis half exposed. Further treatment helped cover the biological prosthesis with granulation tissue. Plastic surgeons evaluated the patient, and VAC therapy was used on the wound for about 15 days.
Gcells Intervention
The Gcells protocol was applied when the wound dimension progressed to about 250cm². The tissue granulation near the ileostomy improved, though it appeared undermined.
Outcome
Gcells protocol proved highly effective in healing and restoring damaged tissues. This progress opens the way for its use in clinical practice for treating and managing acute and chronic wounds and other medical fields needing tissue repair.
Discussion and Conclusion
Gcells protocol has demonstrated efficiency in aiding wound healing, especially for wounds likely to develop from acute to chronic conditions. The working principle of Gcells, using one individual as both the donor and acceptor, reduces complications related to non-autologous implants or micro-grafts. Gcells are flexible and can be used in operating rooms and ambulatories. This innovation is spreading and is currently used in oral-maxillofacial fields, plastic surgery, dermatology, and orthopedics.
Final Thoughts
Gcells offer a promising, efficient, and low-risk innovation in medicine for wound management and healing. The device still needs further testing on subjects with different conditions, but its potential to improve healing of complex wounds is significant. Gcells can reduce the use of expensive devices and offer employment in clinical procedures aiding in wound management.
- Published in Corporate News / Blog
Global Stem Cells Group Enters Training Agreement with Moroccan Hospital
The agreement between Global and CHU Mohammed VI in Marrakech will help enhance staff preparation and clinical offerings in regenerative medicine
MIAMI LAKES, Florida— Stem Cells Training Inc., a subsidiary of the Global Stem Cells Group (GSCG), has announced that it has signed an onsite training agreement with Centre Hospitalo-Universitaire Mohammed VI Marrakech (CHU). GSCG faculty will travel to Marrakech, Morocco to provide this invaluable two-day training to CHU’s staff on February 14 and 15, 2020.
The agreement names the GSCG as CHU’s provider of training for the facility’s regenerative medicine staff. Through the deal, the GSCG will provide preparation and education in the latest regenerative medicine techniques and stem cells protocols, focusing on those utilizing adult stem cells and birth tissue derived from stem cells. In addition to on-site training, the agreement also names the GSCG as CHU’s provider for all necessary equipment to implement regenerative medicine treatments.
The additional training and supplies offered through the agreement will allow CHU to incorporate the industry’s most effective stem cells protocols into the hospital’s treatment options.
The Centre Hospitalo-Universitaire Mohammed VI is a regional leader in quality patient care, student education, physician training, and innovative medical research. Located in Marrakech, CHU provides the bustling city with short-, medium-, and long-range care solutions. Counted among its innovative institutional projects, CHU’s Culture and Health program is committed to making the hospital a more human environment for patients seeking care.
“The agreement between the Global Stem Cells Group and the Centre Hospitalo-Universitaire Mohammed VI Marrakech is an important one for both partners,” said Benito Novas, CEO of the Global Stem Cells Group. “As a university institution, CHU is interested in leading clinical trials to prove the safety and efficacy of regenerative medicine treatment protocols—something the GSCG has a vested interest in. This agreement will allow us to train hospital staff to offer cutting-edge treatments to patients in Morocco while helping stem cells treatments gain more traction globally, making them more viable options for physicians to incorporate into their practices.”
With the newly signed agreement, the GSCG continues to advance its mission to expand its presence across the global, especially focusing on North America and the Middle East. Physicians looking to grow their practices by offering regenerative medicine treatments can benefit from the GSCG’s trainings, which are conveniently held onsite at physicians’ home facilities, no matter where they are located globally.
To learn more about the onsite training opportunities offered by the Global Stem Cells Group, visit https://www.stemcelltraining.net/onsitetraining/. To learn more about the Centre Hospitalo-Universitaire Mohammed VI Marrakech, visit www.chumarrakech.ma.
- Published in Press Releases
ISSCA Announces its 2020 Schedule of Regenerative Medicine Congresses
The group will host six events across the globe, combining networking and education for practitioners of regenerative medicine
MIAMI LAKES, Florida— The International Society for Stem Cell Application (ISSCA), a worldwide leader in regenerative medicine education, has announced its slate of 2020 medical congresses. The group’s calendar includes dates at six locations across the globe
Through its congresses, the ISSCA seeks to create a platform through which physicians can collaborate, share data, initiate discussions, and exchange information that may be directly translated to therapeutic applications. All doctors who want to share their medical research are welcome to attend, and the ISSCA welcomes abstract submissions and reviews them on a continuing basis.
For the second year straight, the main topic discussed at ISSCA’s congresses will be how cellular products derived from birth tissue (allogeneic compounds) have revolutionized the industry by delivering safer, shorter stem cells procedures for practitioners. During each event, lecturers will respond to concerns attendees have about how regenerative medicine advances will satisfy FDA regulations and how new technologies could continue to help patients under new FDA laws.
This year, the ISSCA welcomes three new cities to its agenda—Salvador de Bahia, Brazil; Punta del Este, Uruguay; and Jakarta, Indonesia. The six congresses are as follows:
- May 2020: Mexico City, Mexico
- June 2020: Caracas, Venezuela
- August 2020: Salvador de Bahia, Brazil
- October 2020: Buenos Aires, Argentina
- November 2020: Jakarta, Indonesia
- December 2020: Punta del Este, Uruguay
“We at the ISSCA are looking forward to this year’s calendar of congresses, which will provide invaluable networking and educational opportunities to physicians looking to expand their knowledge on current innovations and best practices concerning regenerative medicine,” noted Benito Novas, ISSCA VP of Public Relations. “Our global congresses expand on ISSCA’s mission to continue to serve as the premier educational resource for physicians across the globe looking to introduce stem cells treatments into their practices.”
To learn more about the ISSCA or to register for an upcoming congress, visit www.issca.us.
- Published in Press Releases


![Secondary-Diabetes[1]](https://www.stemcellsgroup.com/wp-content/uploads/2020/02/Secondary-Diabetes1-460x260_c.png)