Authors:
Michael L. Levy, MD, PhD; John R. Crawford, MD; Nabil Dib, MD; Lev Verkh, PhD; Nikolai Tankovich, MD, PhD; Steven C. Cramer, MD
Background and Purpose
Stroke is a leading cause of long-term disability. Limited treatment options exist for patients with chronic stroke and substantial functional deficits. This study examined the safety and preliminary efficacy of intravenous allogeneic mesenchymal stem cells in this population.
Methods
Entry Criteria
- Ischemic stroke >6 months prior
- Substantial impairment (National Institutes of Health Stroke Scale score ≥6)
- Disability
Treatment Procedure
- Enrollees received a single intravenous dose of allogeneic ischemia-tolerant mesenchymal stem cells.
- Phase 1 used a dose-escalation design (3 tiers, n=5 each).
- Phase 2 was an expanded safety cohort.
Primary and Secondary End Points
- The primary end point was safety over 1 year.
- Secondary end points examined behavioral change.
Results
Phase 1 Findings (n=15)
- Each dose (0.5, 1.0, and 1.5 million cells/kg body weight) was found safe.
Phase 2 Findings (n=21)
- Subjects received 1.5 million cells/kg.
- At baseline, subjects (n=36) averaged 4.2±4.6 years post-stroke, age 61.1±10.8 years, National Institutes of Health Stroke Scale score 8 (6.5–10), and Barthel Index 65±29.
- Two were lost to follow-up, one was withdrawn, and two died (unrelated to study treatment).
Adverse Events
- Of 15 serious adverse events, none was possibly or probably related to study treatment.
- Two mild adverse events were possibly related to study treatment: a urinary tract infection and intravenous site irritation.
- Treatment was safe based on serial exams, electrocardiograms, laboratory tests, and computed tomography scans of chest/abdomen/pelvis.
Behavioral End Points
- All behavioral end points showed significant gains over the 12 months of follow-up.
- Barthel Index scores increased by 6.8±11.4 points at 6 months (P=0.002) and by 10.8±15.5 points at 12 months (P<0.001) post-infusion.
- The proportion of patients achieving excellent functional outcomes (Barthel score ≥95) increased from 11.4% at baseline to 27.3% at 6 months and to 35.5% at 12 months.
Conclusions
Intravenous transfusion of allogeneic ischemia-tolerant mesenchymal stem cells in patients with chronic stroke and substantial functional deficits was safe and suggested behavioral gains. These data support proceeding to a randomized, placebo-controlled study of this therapy in this population.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov
Unique identifier: NCT01297413
Key Words: abdomen, brain ischemia, neuroprotection, pelvis, reperfusion
Mesenchymal Stem Cells as Restorative Therapy for Stroke Patients
Stroke is perennially among the leading causes of human disability and the leading neurological cause of lost disability-adjusted life years. The mean survival after stroke is 6 to 7 years, and indeed more than 85% of patients live past the first year poststroke, many with years of enduring disability. Many restorative therapies are under study to improve outcomes after stroke. Restorative therapies aim to improve patient outcomes by promoting the neural processes underlying behavioral recovery and are distinguished from acute therapies, such as reperfusion or neuroprotection, that aim to reduce initial injury. As such, restorative therapies often have a time window measured in days to months, or in some cases, years.
Mesenchymal Stem Cells (MSC) as a Candidate for Stroke Therapy
Mesenchymal stem cells (MSC), also known as mesenchymal stromal cells, are among the leading restorative therapy candidates. Substantial preclinical data support the safety and efficacy of MSC as a restorative therapy to improve outcomes after stroke. For example, a meta-analysis reported that 44 of 46 preclinical stroke studies found MSC to be superior to placebo, with effect sizes greater than 1.0.
Initial Human Studies of MSC
Initial human studies of MSC (or MSC-like cells) after stroke focused on autologous cell therapies, whereby bone marrow is taken from each patient to produce his/her own MSC batch, and found MSC infusion to be safe. MSC are relatively immunoprivileged given their very low levels of human leukocyte antigen molecule expression, a fact that opens the door to the administration of allogeneic MSC. Allogeneic MSC have been found to be safe without the use of concomitant immunosuppression, and can be manufactured in a manner that enables broad clinical application.
Studies of Allogeneic MSC
Studies of allogeneic MSC (or MSC-like cells) poststroke have focused on early time points (administration 24–48 hours poststroke) or used an invasive procedure to implant cells intracerebrally. Each approach has its relative advantages and disadvantages, and an intravenous method of introducing MSC, if comparably efficacious, might facilitate widespread implementation and also avoid adverse events attributable to invasive procedures.
Current Study Overview
The current study was a phase I/II dose-escalation trial that examined the effects of a single intravenous infusion of allogeneic ischemia-tolerant MSC. The target population was patients with chronic ischemic stroke and substantial functional deficits, a group for whom treatment options remain limited. The primary outcome was safety, based on serial measures of behavior, computed tomography (CT) scans, and laboratory testing. Preliminary estimates of treatment efficacy were also examined.
Inclusion Criteria
- Age ≥18 years
- Ischemic stroke ≥6 months prior, radiologically confirmed at initial diagnosis and at study enrollment
- Severe disability resulting from the index stroke, operationally defined as subject confined to a wheelchair, requiring home nursing care, or needing assistance with activities of daily living
- No substantial improvement in neurological or functional status for the 2 months before study enrollment
- NIHSS score 6–20
- Life expectancy >12 months
- Patient receiving standard of care secondary stroke prevention before enrollment
- Patient or a surrogate able to provide informed consent
- Reasonable expectation that the patient will receive standard posttreatment care and attend all scheduled study visits
- Adequate systemic organ function, specifically:
- Serum aspartate aminotransferase ≤2.5× upper limit of normal
- Serum alanine aminotransferase ≤2.5× upper limit of normal
- Total serum bilirubin ≤1.5× upper limit of normal
- Prothrombin time and partial thromboplastin time ≤1.25× upper limit of normal in subjects not receiving antithrombotic therapy
- Serum albumin ≥3.0 g/dL
- Absolute neutrophil count ≥1500/µL
- Platelet count ≥150,000/µL
- Hemoglobin ≥9.0 g/dL
- Serum creatinine ≤1.5× upper limit of normal
- Serum amylase or lipase ≤1.0× upper limit of normal
Exclusion Criteria
- History of uncontrolled seizure disorder
- History of cancer within the past 5 years, except for localized basal or squamous cell carcinoma
- History of cerebral neoplasm
- Positive for hepatitis B, C, or HIV
- Myocardial infarction within 6 months of study entry
- Presence of any other clinically significant medical or psychiatric condition, or laboratory abnormality, for which study participation would pose a safety risk in the judgment of the Investigator or Sponsor
- Findings on baseline computed tomography suggestive of subarachnoid or intracerebral hemorrhage within the past 12 months
- Participation in another investigational drug or device study in the 3 months before treatment
- History within the past year of drug or alcohol abuse
- Pregnant or lactating, or expectation to become pregnant during the study
- Allergy to bovine or porcine products
NIHSS indicates National Institutes of Health Stroke Scale.
Methods
Study Design
This was a phase I/II multi-center, open-label study that aimed to evaluate the safety and preliminary efficacy of a single intravenous infusion of marrow-derived allogeneic ischemia-tolerant MSC. Entry criteria appear in Table 1 and in sum describe enrollment of adults with radiologically verified chronic stable ischemic stroke and substantial impairment and functional deficits. Patients were followed for one year after MSC infusion. The study made no restrictions on, and did not provide any forms of, medication or therapy (occupational, physical, or speech) during the follow-up year after infusion. All patients signed consent in accordance with local Institutional Review Board approval. This study was approved by the Food and Drug Administration and was registered at clinicaltrials.gov. The data that support the findings of this study are available from the corresponding author on reasonable request.
The study occurred in 2 parts, with part 1 being a dose-escalation study and part 2 being an expanded safety study based on part 1 findings. Part 1 consisted of 3 cohorts (n=5 per cohort) enrolled sequentially in a dose-escalation manner, with subjects receiving one of 3 doses based on body weight, with a maximum dosage of 150 million cells. Cohort 1 received 0.5 million cells/kg of body weight; Cohort 2, 1.0 million cells/kg; and Cohort 3, 1.5 million cells/kg. The dose-escalation plan in part 1 required a review by the Data Safety Monitoring Board once the 5 subjects in Cohort 1 were treated and evaluated through study day 10. If safety was established, Cohort 2 was to proceed at the next highest dose, followed by a similar safety review before escalation to the highest dose in Cohort 3. Part 2 aimed to enroll an additional minimum of 20 subjects at the highest safe dose level determined in part 1. An additional interim review was conducted by the Data Safety Monitoring Board after the first 5 patients were treated in part 2. Detailed stopping rules appear in the online-only Data Supplement (see Stopping Rules and Determination of Maximum Tolerated Dose).
The target dose of 1.5 million cells/kg corresponds to allometric scaling from animal studies. Our meta-analysis of preclinical studies of MSC after experimental ischemic stroke identified 9 rodent studies that transfused MSC using the intravenous route in the post-acute period. In each study, MSC provided substantial behavioral gains (effect sizes >1.0), using doses ranging from 3.6 to 12.4×10^6 MSC/kg body weight (mean dose of 10.1×10^6 MSC/kg). The approach to allometric scaling from animals to humans recommended by the Food and Drug Administration uses a body surface area normalization, which for the mean value in rodents yields a comparable human dose of 1.6×10^6 MSC/kg.
Cell Manufacturing and Shipping
Manufacturing of MSC was performed at the GMP-compliant facility of the sponsor, Stemedica Cell Technologies, Inc (San Diego, CA). MSC were grown from the bone marrow of a single human donor and are from the same batch used in prior preclinical and clinical studies. Cells were grown under low oxygen (5%) conditions. Such ischemia-tolerant MSC have advantages compared with those grown under normoxic conditions, for example, showing higher proliferation rate, expression of stem cell-related genes, production of key cytokines, and migration activity. Cells were harvested at passage 4 and expressed CD105, CD73, and CD90 surface markers, consistent with the International Society for Cellular Therapy definition. Cells were cryopreserved by suspending in Cryostar CS10 freezing medium (BioLife Solutions, Bothell, WA) then stored in the vapor phase of liquid nitrogen. This parent cell bank was then tested for quality control including cell count, viability, appearance, and quantitative polymerase chain reaction for viruses including HIV, Epstein-Barr virus, cytomegalovirus, hepatitis B virus, parvovirus B19, and hepatitis C virus. Cryovials were shipped at ≤-150°C in a vapor phase liquid nitrogen shipper with temperature monitor.
Infusion of Investigational Product
Each site’s pharmacy prepared MSC for infusion per a study-provided protocol. Cryovials (the number of which was based on the dose to be infused) were thawed and MSC were washed in, and then suspended in, Lactated Ringer’s solution at a concentration of 1×10^6 cells/mL using one to three 60 mL syringes. The suspension then underwent final testing before being released for intravenous infusion, consisting of cell count, endotoxin, Gram stain, and review of appearance. Cell count was performed using 0.1% Trypan Blue and a hemacytometer, which also yielded % cell viability. The minimum percent cell viability was required to be ≥70% for the cells to be released. A sample was also sent for subsequent sterility testing. After release by the pharmacy, the final formulation was stored at 2° to 8°C and infused within 8 hours of preparation.
MSC Administration
Before MSC infusion, a 0.1 mL aliquot of the final MSC formulation was injected intradermally; any subject showing a positive reaction (e.g., wheal with erythema) would not be infused. Cells were administered intravenously via metered-dose syringe pump at 2 mL/min. Patients remained in the inpatient telemetry unit for observation until clinically stable.
Patient Assessments
Patients had frequent monitoring until discharged from the telemetry unit. After discharge, patients had safety evaluations on day 2, 3, 4, and 10, then again on month 1, 3, 6, 9, and 12. Adverse events were coded according to the MedDRA adverse event dictionary. The relationship that adverse events had to the investigational product was assessed by the site investigator. Patients were followed for one year using tests of behavior, serology, blood chemistry and cell counts, electrocardiogram, urine, and CT of chest, abdomen, and pelvis. The full schedule of assessments appears in Table SI in the online-only Data Supplement.
Statistics
The primary study endpoint was safety and tolerability, evaluated in all subjects who received any portion of an infusion, and determined by the incidence/severity of adverse events, clinically significant changes on laboratory and imaging tests, vital signs, and physical plus neurological examinations. Four secondary endpoints were scored serially to derive preliminary estimates of efficacy: National Institutes of Health Stroke Scale, Barthel Index (BI), Mini-Mental Status Exam, and Geriatric Depression Scale. For each, the change from baseline was evaluated using the Wilcoxon signed-rank test, with primary analysis of preliminary efficacy being change from baseline to 6 months post-infusion, and analysis including all subjects who received an infusion except for one subject who failed to return after the day 10 visit for all visits (except for month 9 follow-up). For any subject missing 6-month data, 9-month or 12-month data were substituted for this analysis, otherwise missing data were not imputed. Data were analyzed using R statistical software. Given the exploratory nature of this study, sample size was selected as appropriate for detection of any safety concerns in an early phase clinical trial.
Results
Subjects
Of 50 subjects who seemed eligible on prescreening, 36 were enrolled and received treatment from March 14, 2011, to December 15, 2016 (Figure and Table 2). There were 13 subjects enrolled at the University of California, San Diego, 19 subjects at Arizona, and 4 subjects at the University of California, Irvine. Interim safety reviews disclosed no concerns, and so 5 subjects received 0.5×10^6 cells/kg in part 1/Cohort 1, 5 subjects received 1.0×10^6 cells/kg in part 1/Cohort 2, 5 subjects received 1.5×10^6 cells/kg in part 1/Cohort 3, and all 21 subjects in part 2 received 1.5×10^6 cells/kg. For the 15 subjects in part 1, 12 completed the study, 2 died of unrelated causes (coronary artery disease 6 months post-infusion and sepsis 1 month after infusion), and 1 was lost to follow-up after day 10 (reappearing only for the month 9 follow-up visit). For the 21 subjects in part 2, 19 completed the study, 1 was lost to follow-up after month 6, and 1 was withdrawn by the site PI after month 6 due to treatment with another investigational product. Of the 36 subjects enrolled, the planned dose was delivered within 2 mL (i.e., within 2×10^6 cells) of the target in 26 subjects, whereas in 10 subjects a median of 7.6 (interquartile range, 4.4–10.25) mL (i.e., 7.6×10^6 cells) was not infused as planned, which represented a median of 6.5% (5.3–9.8) of the intended dose. A total of 179 protocol deviations were reported, mainly related to scheduling study visits or study testing (Table SII in the online-only Data Supplement).
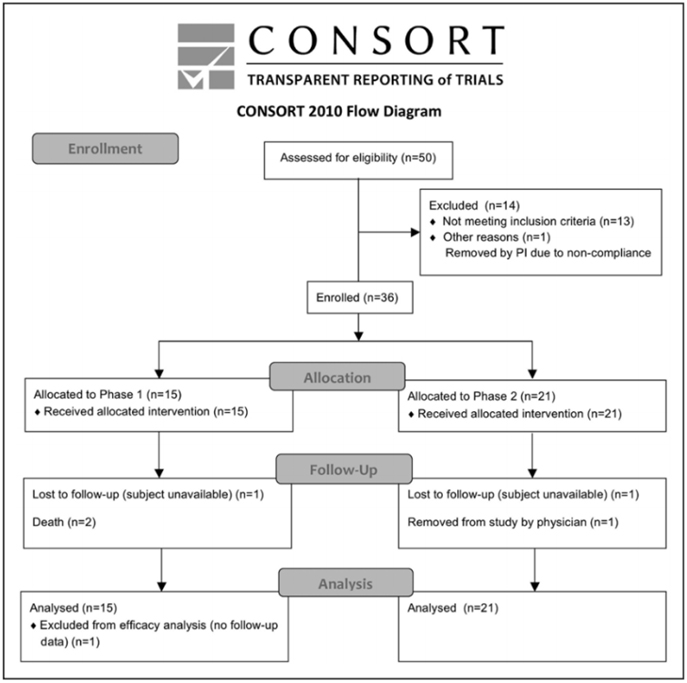
Figure. CONSORT diagram.
Table 2. Baseline Subject Characteristics
| Characteristic | Cohort 1 | Cohort 2 | Cohort 3 | Total |
|---|---|---|---|---|
| n | 5 | 5 | 5 | 21 |
| Sex | ||||
| – Male | 5 (100%) | 4 (80%) | 4 (80%) | 14 (66.67%) |
| – Female | 0 (0%) | 1 (20%) | 1 (20%) | 7 (33.33%) |
| Age, y | 50.8 ± 9.8 | 56.8 ± 11.1 | 68.8 ± 11.58 | 62.8 ± 9.2 |
| [40—62] | [39—69] | [53—84] | [51—83] | |
| Race | ||||
| – White | 4 (80%) | 3 (60%) | 5 (100%) | 17 (80.95%) |
| – Asian | 0 (0%) | 1 (20%) | 0 (0%) | 0 (0%) |
| – American Indian/Alaskan Native | 0 (0%) | 1 (20%) | 0 (0%) | 0 (0%) |
| – Native Hawaiian/Pacific Islander | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| – Black | 1 (20%) | 0 (0%) | 0 (0%) | 1 (4.76%) |
| – Other | 0 (0%) | 0 (0%) | 0 (0%) | 3 (14.29%) |
| Ethnicity | ||||
| – Hispanic or Latino | 0 (0%) | 0 (0%) | 0 (0%) | 2 (9.52%) |
| – Non-Hispanic or Non-Latino | 5 (100%) | 5 (100%) | 5 (100%) | 19 (90.48%) |
| Living situation | ||||
| – At home | 5 (100%) | 5 (100%) | 3 (60%) | 19 (90.48%) |
| – In a living facility | 0 (0%) | 0 (0%) | 2 (40%) | 2 (9.52%) |
| Time from stroke to infusion, y | 1.6 ± 0.9 | 7.7 ± 5.0 | 4.1 ± 2.2 | 4.0 ± 5.0 |
| [0.6—2.9] | [1.1—14.5] | [1.7—7.0] | [0.7—24.8] | |
| Total | 5 | 5 | 5 | 36 |
Safety
A total of 15 serious adverse events were reported. These were wide-ranging in nature, for example, infections, vascular disorders, and pain syndromes (for full details, see Table SIII in the online-only Data Supplement). All serious adverse events were deemed unrelated or unlikely related to the investigational product. A total of 109 adverse events were reported, of which 2, both mild, were considered by the site investigator to be possibly related to the investigational product: one urinary tract infection and one report of intravenous site irritation. Both adverse events recovered completely.
Study testing disclosed no safety concerns. No subject showed a preinfusion positive reaction to intradermal testing. Serial physical exams and blood testing did not disclose any significant findings. Only one of the serial electrocardiograms was thought to have clinically significant findings, in a subject with moderate intraventricular conduction delay, only at the 1-month follow-up visit. Similarly, across serial CT scans of the chest, abdomen, and pelvis, only one was considered clinically significant, a soft tissue density in the anterior abdominal wall seen at 6-months that was stable when reimaged at 12-months.
Behavioral Effects
Across all subjects, improvements were seen in National Institutes of Health Stroke Scale, BI, Mini-Mental Status Exam, and Geriatric Depression Scale scores at both the 6-month and the 12-month follow-up visits (Table 3). These were statistically significant, generally stable over time, and clinically modest in magnitude. Most findings would survive correction for multiple comparisons. Changes in the BI suggest clinical utility, with a 6.8 point gain by 6-months that grew to a 10.8 point gain by 12-months post-infusion (P<0.001), and with the proportion of patients achieving excellent functional outcome (Barthel score =95) increasing from 11.4% (4/35) at baseline to 9/33 (27.3%) at 6-months to 35.5% (11/31) at 12-months.
Discussion
Stroke is a major cause of human disability. This can be reduced by acute therapies that are introduced in the early hours poststroke to reduce initial injury, and by restorative therapies that are introduced days, months, or years poststroke to promote neural repair. Allogeneic MSC show substantial favorable effects in preclinical studies, including when introduced via the intravenous route. The current study found a single intravenous infusion of allogeneic MSC to be safe and potentially associated with functional improvement.
The current study is the largest trial of intravenous MSC in patients with chronic stroke and the first to evaluate allogeneic MSC therapy in this population. It is also the first human stroke study to evaluate MSC grown under hypoxic conditions, which favorably affects cell proliferation, gene expression, cytokine production, and migration. Intravenous infusion of MSC was found to be safe in 36 patients who had chronic stroke with substantial functional deficits. Across 3 escalating doses, treatment-related adverse events were infrequent, mild, and transient. Serial assessments of exam, laboratory testing, electrocardiogram, and CT scans of chest/abdomen/pelvis disclosed no safety concerns, with limited subject dropout. These results are consistent with the overall excellent safety record that MSC have in clinical trials of human subjects across numerous non-cerebrovascular diagnoses and in stroke trials.
Patients with stroke in the chronic stage generally show functional decline; however, enrollees in the current study showed 12 months of continued functional improvement. In general, recovery from stroke-related deficits shows a bimodal time course. Initially, most stroke survivors show some degree of spontaneous recovery, for example, during the initial months for the motor system. Within a year of stroke onset, however, a significant decline in function is commonly seen. This is significant given that few treatment options are available to improve function in patients in the chronic phase of stroke. In the current study, behavioral gains were seen, though were modest in magnitude. However, a 2-point improvement in the National Institutes of Health Stroke Scale score in the setting of chronic stroke, if verified in a larger controlled study, might be regarded as important. Also, the mean gain in BI from baseline grew to 10.8 points by 12 month-poststroke (P<0.001), higher than the BI minimal clinically important difference of 9.25 points. Furthermore, the proportion of patients with an excellent functional outcome (BI score =95) increased from 11.4% at baseline to 27.3% at 6-months and to 35.5% at 12-months. This 12-month period of continued functional improvement is consistent with preclinical studies examining the distribution of systemically administered MSC: intravenous MSC given early after stroke initially localize to lungs then spleen, then increase within the region of brain ischemia, and by 30 days poststroke are concentrated in the peri-infarct region. At one year, most surviving MSC are in the peri-infarct region, with very few present in other organs. Patients also showed significant improvement in the Mini-Mental Status Exam and Geriatric Depression Scale, changes that were largely sustained at 12 months post-infusion, suggesting that MSC have broad effects on brain function. These findings require verification in a larger, controlled study but raise hope that this intervention could improve functional status in the chronic stroke setting. Future studies might also incorporate modality-specific outcome measures to provide more granular assessments of behavioral gains in individual neural systems.
Table 3. Behavioral Change Over Time
| Measure | Baseline | Change to 6 mo | Change to 12 mo |
|---|---|---|---|
| Mini-Mental Status Exam score | 24.2±6.0 (n=35) | 1.8±2.8 (n=32) | 1.3±2.7 (n=31) |
| P Value | <0.001 | 0.017 | |
| NIHSS score | 8 [6.5 to 10] | -1 [-2.25 to 0] | -2 [-3.5 to -0.5] |
| P Value | <0.001 | <0.001 | |
| Geriatric depression scale score | 5.1±3.5 (n=35) | -1.6±3.8 (n=32) | -1.4±3.8 (n=31) |
| P Value | 0.015 | ||
| Barthel Index (score) | 65±28.7 (n=35) | 6.8±11.4 (n=33) | 10.8±15.5 (n=31) |
| P Value | 0.002 | <0.001 | |
| Barthel Index (% =95) | |||
| Proportion at baseline | 4 (11.4%) | 9 (27.3%) | 11 (35.5%) |
| P Value | 0.015 | 0.01 |
Meta-analysis of MSC Effects in Animals with Experimental Ischemic Stroke
Meta-analysis conducted on MSC effects in animals with experimental ischemic stroke demonstrated substantial and sustained effect sizes, even after adjusting for potential publication bias. This effect was consistent across various factors such as species, delivery route, timing of administration post-stroke, and dosage. Preclinical studies have shown that MSC introduction up to 1 month or 4 to 6 weeks post-infarct yields promising results.
Bidirectional Translation: Bedside Experience Informing Preclinical Studies
Findings from patients many months post-stroke (refer to Table 2) underscore the necessity for bidirectional translation. This approach involves translating bedside experiences into insights that inform and refine preclinical studies. This iterative process enhances the applicability and efficacy of future treatments.
Study Strengths
Population with Substantial Functional Deficits
The study enrolled patients in the chronic stage of stroke, a demographic numbering in the millions worldwide, with limited treatment options.
Utilization of Allogeneic MSC
The study utilized allogeneic MSC due to their relatively immunoprivileged nature, eliminating the need for immunosuppression. This approach enables broader implementation within the stroke population.
Safety Evaluation
A dose-escalation design evaluated safety aspects rigorously. Cell culture was limited to 4 passages to optimize MSC features crucial for efficacy.
Study Limitations
Lack of Control Group
Since the study focused primarily on safety, the absence of a control group complicates the interpretation of observed behavioral improvements (refer to Table 3).
Mechanism of Action
The mechanism of action underlying cell therapies improving outcomes in the chronic phase remains unexplored. Future trials should investigate mechanisms like growth factor release, anti-inflammatory effects, and exosome involvement.
Conclusion: Implications for Future Research
This study establishes the safety of intravenous allogeneic MSC in chronic stroke patients with substantial functional deficits. While suggesting functional benefits, further verification through controlled studies is imperative. These findings advocate for continued research into intravenous allogeneic MSC for chronic stroke, including mechanistic exploration.
To improve the on-page SEO for your article and add H2 headings for references, here’s a structured approach:
References
- Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53.
- Johnston SC, Hauser SL. Neurological disease on the global agenda. Ann Neurol. 2008;64:A11–A12.
- Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215.
- Lin DJ, Finklestein SP, Cramer SC. New directions in treatments targeting stroke recovery. Stroke. 2018;49:3107–3114. doi: 10.1161/STROKEAHA.118.021359
- Cramer SC. Repairing the human brain after stroke. II. Restorative therapies. Ann Neurol. 2008;63:549–560. doi: 10.1002/ana.21412
- Wolf SL, Winstein CJ, Miller JP, et al; EXCITE Investigators. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095
- McCabe J, Monkiewicz M, Holcomb J, et al. Comparison of robotics, functional electrical stimulation, and motor learning methods for treatment of persistent upper extremity dysfunction after stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2015;96:981–990. doi: 10.1016/j.apmr.2014.10.022
- Dodakian L, McKenzie AL, Le V, et al. A home-based telerehabilitation program for patients with stroke. Neurorehabil Neural Repair. 2017;31:923–933. doi: 10.1177/1545968317733818
- Ward NS, Brander F, Kelly K. Intensive upper limb neurorehabilitation in chronic stroke: outcomes from the Queen Square programme. J Neurol Neurosurg Psychiatry. 2019;90:498–506. doi: 10.1136/jnnp-2018-319954
- Vu Q, Xie K, Eckert M, et al. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology. 2014;82:1277–1286. doi: 10.1212/WNL.0000000000000278
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501
- Honmou O, Houkin K, Matsunaga T, et al. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134(pt 6):1790–1807. doi: 10.1093/brain/awr063
- Bhasin A, Srivastava MV, Kumaran SS, et al. Autologous mesenchymal stem cells in chronic stroke. Cerebrovasc Dis Extra. 2011;1:93–104. doi: 10.1159/000333381
- Le Blanc K, Tammik C, Rosendahl K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896.
- Lalu MM, McIntyre L, Pugliese C, et al; Canadian Critical Care Trials Group. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559. doi: 10.1371/journal.pone.0047559
- Hess DC, Wechsler LR, Clark WM, et al. Safety and efficacy of multipotent adult progenitor cells in acute ischemic stroke (MASTERS): a randomized, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16:360–368. doi: 10.1016/S1474-4422(17)30046-7
- Steinberg GK, Kondziolka D, Wechsler LR, et al. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke. 2016;47:1817–1824. doi: 10.1161/STROKEAHA.116.012995
- Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. 2005. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm078932.pdf. Accessed June 22, 2019.
- Luger D, Lipinski MJ, Westman PC, et al. Intravenously delivered mesenchymal stem cells: systemic anti-inflammatory effects improve left ventricular dysfunction in acute myocardial infarction and ischemic cardiomyopathy. Circ Res. 2017;120:1598–1613. doi: 10.1161/CIRCRESAHA.117.310599
- Harach T, Jammes F, Muller C, et al. Administrations of human adult ischemia-tolerant mesenchymal stem cells and factors reduce amyloid beta pathology in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2017;51:83–96. doi: 10.1016/j.neurobiolaging.2016.11.009
- Butler J, Epstein SE, Greene SJ, et al. Intravenous allogeneic mesenchymal stem cells for nonischemic cardiomyopathy: safety and efficacy results of a phase II-a randomized trial. Circ Res. 2017;120:332–340. doi: 10.1161/CIRCRESAHA.116.309717
- Vertelov G, Kharazi L, Muralidhar MG, et al. High targeted migration of human mesenchymal stem cells grown in hypoxia is associated with enhanced activation of RhoA. Stem Cell Res Ther. 2013;4:5. doi: 10.1186/scrt153
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905
- Devine SM. Mesenchymal stem cells: will they have a role in the clinic? J Cell Biochem Suppl. 2002;38:73–79.
- Hilfiker A, Kasper C, Hass R, Haverich A. Mesenchymal stem cells and progenitor cells in connective tissue engineering and regenerative medicine: is there a future for transplantation? Langenbecks Arch Surg. 2011;396:489–497. doi: 10.1007/s00423-011-0762-